Deck 2: Life's Chemical Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/67
Play
Full screen (f)
Deck 2: Life's Chemical Bases
1
The atomic number of an atom refers to its ____.
A) mass or weight
B) number of protons
C) number of protons and neutrons
D) number of neutrons
E) number of electrons
A) mass or weight
B) number of protons
C) number of protons and neutrons
D) number of neutrons
E) number of electrons
B
2
Figure 2.5B
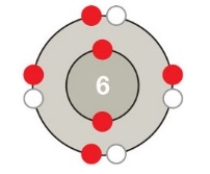
Which atom is depicted in the accompanying figure?
A) Hydrogen
B) Helium
C) Carbon
D) Nitrogen
E) Oxygen

Which atom is depicted in the accompanying figure?
A) Hydrogen
B) Helium
C) Carbon
D) Nitrogen
E) Oxygen
C
3
What is the smallest unit of an element that retains the properties of that element?
A) Atom
B) Compound
C) Orbital
D) Molecule
E) Mixture
A) Atom
B) Compound
C) Orbital
D) Molecule
E) Mixture
A
4
The ____ of an atom have no charge.
A) electrons
B) protons
C) neutrons
D) ions
E) nuclei
A) electrons
B) protons
C) neutrons
D) ions
E) nuclei

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
5
Figure 2.5 C
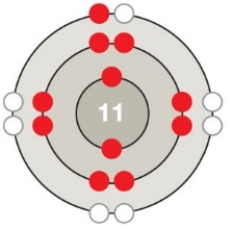
Which of the following is depicted in the accompanying figure?
A) Hydrogen atom
B) Sodium atom
C) Helium ion
D) Chlorine ion
E) Oxygen molecule

Which of the following is depicted in the accompanying figure?
A) Hydrogen atom
B) Sodium atom
C) Helium ion
D) Chlorine ion
E) Oxygen molecule

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
6
Tracers are elements that ____.
A) are used in minute amounts in plants
B) can be monitored during biochemical reactions
C) must be inert
D) have an unbalanced electrical charge
E) must have a stable nucleus
A) are used in minute amounts in plants
B) can be monitored during biochemical reactions
C) must be inert
D) have an unbalanced electrical charge
E) must have a stable nucleus

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
7
An atom can get rid of vacancies by participating in a ___________.
A) cell bond
B) physical bond
C) chemical bond
D) magnetic bond
E) electric bond
A) cell bond
B) physical bond
C) chemical bond
D) magnetic bond
E) electric bond

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
8
Figure 2.5A
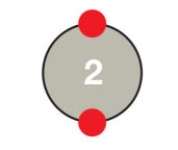
Based on its outer shell,the atom in the accompanying figure would be characterized as ____.
A) very stable
B) somewhat stable
C) somewhat unstable
D) very unstable
E) radioactive

Based on its outer shell,the atom in the accompanying figure would be characterized as ____.
A) very stable
B) somewhat stable
C) somewhat unstable
D) very unstable
E) radioactive

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
9
In the chemical shorthand,14C,the 14 represents the number of ____.
A) excess neutrons
B) protons plus neutrons
C) electrons
D) protons plus electrons
E) radioactive particles
A) excess neutrons
B) protons plus neutrons
C) electrons
D) protons plus electrons
E) radioactive particles

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
10
The nucleus of an atom contains ____.
A) neutrons and protons
B) neutrons and electrons
C) protons and electrons
D) protons only
E) neutrons only
A) neutrons and protons
B) neutrons and electrons
C) protons and electrons
D) protons only
E) neutrons only

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
11
A(n)__________ is a strong mutual attraction between ions of opposite charge.
A) ionic bond
B) molecular bond
C) covalent bond
D) polar covalent bond
E) magnetic bond
A) ionic bond
B) molecular bond
C) covalent bond
D) polar covalent bond
E) magnetic bond

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
12
When can we say that atom has no vacancy,or the atom is full?
A) An atom's outer shell is filled with electrons
B) An atom's inner shell is filled with electrons
C) An atom's outer shell is filled with neutrons
D) An atom's outer shell is filled with protons
E) An atom's inner shell is filled with protons
A) An atom's outer shell is filled with electrons
B) An atom's inner shell is filled with electrons
C) An atom's outer shell is filled with neutrons
D) An atom's outer shell is filled with protons
E) An atom's inner shell is filled with protons

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
13
How does the energy of an electron relate with the distance from the nucleus?
A) The closer an electron is from the nucleus, the greater its energy.
B) The farther an electron is from the nucleus, the greater its energy.
C) The farther a proton is from the nucleus, the greater the electron's energy.
D) The closer a proton is from the nucleus, the greater the electron's energy.
E) The closer a neutron is from the nucleus, the greater the electron's energy.
A) The closer an electron is from the nucleus, the greater its energy.
B) The farther an electron is from the nucleus, the greater its energy.
C) The farther a proton is from the nucleus, the greater the electron's energy.
D) The closer a proton is from the nucleus, the greater the electron's energy.
E) The closer a neutron is from the nucleus, the greater the electron's energy.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
14
The mass number of an atom is determined by the combined masses of its ____.
A) neutrons and protons
B) neutrons and electrons
C) protons and electrons
D) protons, neutrons, and electrons
E) neutrons, nucleus, and electrons
A) neutrons and protons
B) neutrons and electrons
C) protons and electrons
D) protons, neutrons, and electrons
E) neutrons, nucleus, and electrons

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
15
The ____ of an atom have a negative charge.
A) nuclei
B) protons
C) neutrons
D) ions
E) electrons
A) nuclei
B) protons
C) neutrons
D) ions
E) electrons

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
16
Isotopes of atoms ____.
A) have the same number of neutrons but a different number of protons
B) behave the same chemically and biologically from other isotopes
C) are the same physically and biologically but differ from other isotopes chemically
D) have the same number of protons but a different number of electrons
E) are produced when atoms lose electrons
A) have the same number of neutrons but a different number of protons
B) behave the same chemically and biologically from other isotopes
C) are the same physically and biologically but differ from other isotopes chemically
D) have the same number of protons but a different number of electrons
E) are produced when atoms lose electrons

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
17
The radioisotope 14C can be used as a research tracer because it ____.
A) decays to 12C
B) has a different number of protons than 12C
C) has fewer neutrons than 12C
D) behaves the same chemically as 12C
E) has six carbons and six neutrons
A) decays to 12C
B) has a different number of protons than 12C
C) has fewer neutrons than 12C
D) behaves the same chemically as 12C
E) has six carbons and six neutrons

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
18
Isotopes of an element are differentiated by their ____.
A) atomic weight
B) number of orbital shells
C) element name
D) mass number
E) electron profile
A) atomic weight
B) number of orbital shells
C) element name
D) mass number
E) electron profile

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
19
All isotopes of an element have a different number of ____.
A) electrons
B) protons
C) neutrons
D) orbital shells
E) atoms
A) electrons
B) protons
C) neutrons
D) orbital shells
E) atoms

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
20
Which substance is not an element?
A) Chlorine
B) Oxygen
C) Carbon
D) Water
E) Hydrogen
A) Chlorine
B) Oxygen
C) Carbon
D) Water
E) Hydrogen

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
21
"Acidic" is an appropriate description for four of the following.Which one is the exception?
A) Excess hydrogen ions
B) The contents of the stomach
C) Magnesium hydroxide
D) HCl
E) A pH less than 7
A) Excess hydrogen ions
B) The contents of the stomach
C) Magnesium hydroxide
D) HCl
E) A pH less than 7

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
22
A salt will dissolve in water to form ____.
A) acids
B) only hydrogen and oxygen bonds
C) ions other than H+ and OH-
D) bases
E) buffers
A) acids
B) only hydrogen and oxygen bonds
C) ions other than H+ and OH-
D) bases
E) buffers

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
23
The column of water extending in tubes from plant roots to leaves is maintained by ____.
A) hydrophilic interactions
B) ionic bonds
C) covalent bonds
D) hydrophobic interactions
E) cohesion between water molecules
A) hydrophilic interactions
B) ionic bonds
C) covalent bonds
D) hydrophobic interactions
E) cohesion between water molecules

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
24
In ____ bonds,both atoms exert the same pull on shared electrons.
A) triple covalent
B) polar covalent
C) double covalent
D) nonpolar covalent
E) coordinate covalent
A) triple covalent
B) polar covalent
C) double covalent
D) nonpolar covalent
E) coordinate covalent

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
25
Atoms with a(n)____ are more likely to form chemical bonds.
A) filled outer orbital shell
B) unfilled outer orbital shell
C) filled inner orbital shell
D) unfilled inner orbital shell
E) large number of orbital shells
A) filled outer orbital shell
B) unfilled outer orbital shell
C) filled inner orbital shell
D) unfilled inner orbital shell
E) large number of orbital shells

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
26
When exposed to water,sodium chloride (NaCl)____.
A) dissolves into Na+ and Cl- ions
B) crystallizes into a solid
C) dissolves into Na- and Cl+ ions
D) crystallizes into a liquid
E) forms a hydrophobic compound
A) dissolves into Na+ and Cl- ions
B) crystallizes into a solid
C) dissolves into Na- and Cl+ ions
D) crystallizes into a liquid
E) forms a hydrophobic compound

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
27
Nitrogen,with an atomic number of 7,has ____ electron(s)in the first energy level and ____ electrons in the second energy level.
A) one; six
B) two; five
C) three; four
D) four; three
E) five; two
A) one; six
B) two; five
C) three; four
D) four; three
E) five; two

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
28
Which statement is false?
A) A molecule must be made of at least two atoms.
B) Compounds are made of elements.
C) Two atoms of oxygen make a molecule of oxygen.
D) Chemical bonds form between molecules of solute and solvent.
E) Elements are found in compounds and molecules.
A) A molecule must be made of at least two atoms.
B) Compounds are made of elements.
C) Two atoms of oxygen make a molecule of oxygen.
D) Chemical bonds form between molecules of solute and solvent.
E) Elements are found in compounds and molecules.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
29
Water is important to the interactions of biological molecules because it ____.
A) is a good buffer
B) destabilizes temperature
C) is a poor solvent for polar and ionic substances
D) has weak cohesive properties
E) promotes hydrophilic interactions
A) is a good buffer
B) destabilizes temperature
C) is a poor solvent for polar and ionic substances
D) has weak cohesive properties
E) promotes hydrophilic interactions

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
30
A molecule consists of ____.
A) radioactive compounds
B) two or more atoms of the same element
C) electrically charged elements
D) elements with one or more extra neutrons
E) atoms held together by chemical bonds
A) radioactive compounds
B) two or more atoms of the same element
C) electrically charged elements
D) elements with one or more extra neutrons
E) atoms held together by chemical bonds

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
31
The bond in table salt (NaCl)is ____.
A) polar
B) ionic
C) covalent
D) double
E) nonpolar
A) polar
B) ionic
C) covalent
D) double
E) nonpolar

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
32
Figure 2.11B
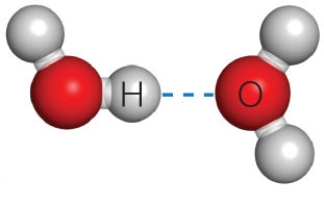
The dashed line in the accompanying figure represents a(n)____.
A) covalent bond
B) ionic bond
C) hydrogen bond
D) polar covalent bond
E) hydrophobic interaction

The dashed line in the accompanying figure represents a(n)____.
A) covalent bond
B) ionic bond
C) hydrogen bond
D) polar covalent bond
E) hydrophobic interaction

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
33
A hydrogen bond is an attraction between a(n)____ hydrogen atom and another atom taking part in ____.
A) covalently bonded; the same polar covalent bond
B) ionically bonded; the same polar covalent bond
C) covalently bonded; a separate polar covalent bond
D) ionically bonded; a separate nonpolar covalent bond
E) nonpolar covalently bonded; a separate nonpolar covalent bond
A) covalently bonded; the same polar covalent bond
B) ionically bonded; the same polar covalent bond
C) covalently bonded; a separate polar covalent bond
D) ionically bonded; a separate nonpolar covalent bond
E) nonpolar covalently bonded; a separate nonpolar covalent bond

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
34
The most likely reason that glucose dissolves in water is that it is ____.
A) an ionic compound
B) a polysaccharide
C) polar and forms many hydrogen bonds with the water molecules
D) an extremely unstable molecule
E) highly nonpolar
A) an ionic compound
B) a polysaccharide
C) polar and forms many hydrogen bonds with the water molecules
D) an extremely unstable molecule
E) highly nonpolar

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
35
What is a buffer?
A) A substance that releases hydrogen ions in water
B) A substance that accepts hydrogen ions in water
C) A substance that accepts oxygen ions in water
D) A set of chemicals that keep the pH of a solution stable
E) A substance that releases oxygen ions in water
A) A substance that releases hydrogen ions in water
B) A substance that accepts hydrogen ions in water
C) A substance that accepts oxygen ions in water
D) A set of chemicals that keep the pH of a solution stable
E) A substance that releases oxygen ions in water

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
36
The solvent,cohesive,and temperature stabilization properties of water are primarily due to its ____.
A) ability to promote hydrophilic interactions
B) ionic bonds
C) hydrogen bonds
D) ability to promote hydrophobic interactions
E) nonpolar nature
A) ability to promote hydrophilic interactions
B) ionic bonds
C) hydrogen bonds
D) ability to promote hydrophobic interactions
E) nonpolar nature

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
37
The slight positive charge of a hydrogen atom in one water molecule is drawn to the slight negative charge of an oxygen atom in another.This interaction is known as a(n)__________.
A) oxygen bond
B) water bond
C) hydrogen bond
D) covalent polarity bond
E) magnetic bond
A) oxygen bond
B) water bond
C) hydrogen bond
D) covalent polarity bond
E) magnetic bond

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
38
Which bond can break most easily?
A) Ionic bond
B) Covalent bonds
C) Polar covalent bond
D) Hydrogen bond
E) Magnetic bond
A) Ionic bond
B) Covalent bonds
C) Polar covalent bond
D) Hydrogen bond
E) Magnetic bond

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
39
In covalent bonds,____.
A) atoms share electrons
B) atoms give up electrons
C) atoms accept electrons
D) electrons cannot be shared equally
E) electrons are always shared equally
A) atoms share electrons
B) atoms give up electrons
C) atoms accept electrons
D) electrons cannot be shared equally
E) electrons are always shared equally

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
40
Atoms can form ____ in order to achieve a full outer orbital shell.
A) ions
B) covalent bonds
C) H bonds
D) ions and covalent bonds
E) ions and H bonds
A) ions
B) covalent bonds
C) H bonds
D) ions and covalent bonds
E) ions and H bonds

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
41
A solution with a pH of 9 has ____ times fewer hydrogen ions than a solution with a pH of 6.
A) two
B) four
C) 10
D) 100
E) 1,000
A) two
B) four
C) 10
D) 100
E) 1,000

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
42
Classification. The following are important terms relating to water's special properties. Answer the questions below by matching the descriptions with the most appropriate word.
a.hydrophobic
b.hydrophilic
c.salt
d.solute
e.Solvent
A liquid that dissolves other substances
a.hydrophobic
b.hydrophilic
c.salt
d.solute
e.Solvent
A liquid that dissolves other substances

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
43
14C is a radioactive isotope,and it turns into ____________________ when it decays.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
44
Classification. The following are types of chemical bonds. Answer the questions below by matching the descriptions with the most appropriate bond type.
a.hydrogen
b.ionic
c.covalent
d.polar covalent
e.double bond
The bond that breaks when salts dissolve in water
a.hydrogen
b.ionic
c.covalent
d.polar covalent
e.double bond
The bond that breaks when salts dissolve in water

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
45
The ability of a solution to resist changes in pH depends on its ____________________ capacity.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
46
Blood pH is kept near a value of 7.3-7.5 because of ____.
A) salts
B) buffers
C) acids
D) bases
E) water
A) salts
B) buffers
C) acids
D) bases
E) water

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
47
Classification. The various energy levels in an atom of magnesium (24Mg) have different numbers of electrons. Use the numbers below to answer the following questions.
-The number of electrons in the second energy level
A)1
B)2
C)3
D)6
E)8
-The number of electrons in the second energy level
A)1
B)2
C)3
D)6
E)8

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
48
Classification. The following are types of chemical bonds. Answer the questions below by matching the descriptions with the most appropriate bond type.
-The bond between the oxygen atoms of oxygen gas (O2)
A)hydrogen
B)ionic
C)covalent
D)polar covalent
E)double bond
-The bond between the oxygen atoms of oxygen gas (O2)
A)hydrogen
B)ionic
C)covalent
D)polar covalent
E)double bond

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
49
Classification. The following are types of chemical bonds. Answer the questions below by matching the descriptions with the most appropriate bond type.
a.hydrogen
b.ionic
c.covalent
d.polar covalent
e.double bond
The bond between the atoms of table salt (NaCl)
a.hydrogen
b.ionic
c.covalent
d.polar covalent
e.double bond
The bond between the atoms of table salt (NaCl)

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
50
Classification. The following are important terms relating to water's special properties. Answer the questions below by matching the descriptions with the most appropriate word.
a.hydrophobic
b.hydrophilic
c.salt
d.solute
e.Solvent
Property of NaCl that enables it to dissolve in water
a.hydrophobic
b.hydrophilic
c.salt
d.solute
e.Solvent
Property of NaCl that enables it to dissolve in water

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
51
Classification. The following are types of chemical bonds. Answer the questions below by matching the descriptions with the most appropriate bond type.
a.hydrogen
b.ionic
c.covalent
d.polar covalent
e.double bond
A bond in which connected atoms unequally share electrons
a.hydrogen
b.ionic
c.covalent
d.polar covalent
e.double bond
A bond in which connected atoms unequally share electrons

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
52
Tracers allow scientists to track a molecule through a biochemical process by replacing an atom in that molecule with its _________.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
53
The sharing of two pairs of electrons between two atoms is called a(n)____________________.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
54
The predictable rate of ____________________ allows scientists to estimate the age of a rock or fossil by examining its isotope content.

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
55
Classification. The following are types of chemical bonds. Answer the questions below by matching the descriptions with the most appropriate bond type.
a.hydrogen
b.ionic
c.covalent
d.polar covalent
e.double bond
The bond type holding several molecules of water together
a.hydrogen
b.ionic
c.covalent
d.polar covalent
e.double bond
The bond type holding several molecules of water together

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
56
Classification. The various energy levels in an atom of magnesium (24Mg) have different numbers of electrons. Use the numbers below to answer the following questions.
-The number of electrons in the third energy level
A)1
B)2
C)3
D)6
E)8
-The number of electrons in the third energy level
A)1
B)2
C)3
D)6
E)8

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
57
Classification. The various energy levels in an atom of magnesium (24Mg) have different numbers of electrons. Use the numbers below to answer the following questions.
-The number of electrons in the first energy level
A)1
B)2
C)3
D)6
E)8
-The number of electrons in the first energy level
A)1
B)2
C)3
D)6
E)8

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
58
Classification. The following are types of chemical bonds. Answer the questions below by matching the descriptions with the most appropriate bond type.
a.hydrogen
b.ionic
c.covalent
d.polar covalent
e.double bond
A bond in which connected atoms share electrons
a.hydrogen
b.ionic
c.covalent
d.polar covalent
e.double bond
A bond in which connected atoms share electrons

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
59
Classification. The following are important terms relating to water's special properties. Answer the questions below by matching the descriptions with the most appropriate word.
a.hydrophobic
b.hydrophilic
c.salt
d.solute
e.Solvent
A compound that releases ions when dissolved in water
a.hydrophobic
b.hydrophilic
c.salt
d.solute
e.Solvent
A compound that releases ions when dissolved in water

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
60
Classification. The following are important terms relating to water's special properties. Answer the questions below by matching the descriptions with the most appropriate word.
a.hydrophobic
b.hydrophilic
c.salt
d.solute
e.Solvent
NaCl becomes this in solution
a.hydrophobic
b.hydrophilic
c.salt
d.solute
e.Solvent
NaCl becomes this in solution

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
61
Classification. The following are important terms relating to acids and bases. Answer the questions below by matching the descriptions with the most appropriate word.
a.pH
b.acid
c.base
d.Buffer
Toothpaste
a.pH
b.acid
c.base
d.Buffer
Toothpaste

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
62
Classification. The following are important terms relating to water's special properties. Answer the questions below by matching the descriptions with the most appropriate word.
a.hydrophobic
b.hydrophilic
c.salt
d.solute
e.Solvent
Property of nonpolar compounds
a.hydrophobic
b.hydrophilic
c.salt
d.solute
e.Solvent
Property of nonpolar compounds

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
63
Classification. The following are important terms relating to acids and bases. Answer the questions below by matching the descriptions with the most appropriate word.
a.pH
b.acid
c.base
d.Buffer
Set of chemicals that stabilize pH
a.pH
b.acid
c.base
d.Buffer
Set of chemicals that stabilize pH

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
64
Classification. The following are important terms relating to acids and bases. Answer the questions below by matching the descriptions with the most appropriate word.
a.pH
b.acid
c.base
d.Buffer
Lemon juice
a.pH
b.acid
c.base
d.Buffer
Lemon juice

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
65
Classification. The following are important terms relating to acids and bases. Answer the questions below by matching the descriptions with the most appropriate word.
-Measure of H+ in a fluid
A)pH
B)acid
C)base
D)Buffer
-Measure of H+ in a fluid
A)pH
B)acid
C)base
D)Buffer

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
66
Classification. The following are important terms relating to acids and bases. Answer the questions below by matching the descriptions with the most appropriate word.
-Substance that accepts,but does not release,H+
A)pH
B)acid
C)base
D)Buffer
-Substance that accepts,but does not release,H+
A)pH
B)acid
C)base
D)Buffer

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck
67
Classification. The following are important terms relating to acids and bases. Answer the questions below by matching the descriptions with the most appropriate word.
-Substance that releases,but does not accept,H+
A)pH
B)acid
C)base
D)Buffer
-Substance that releases,but does not accept,H+
A)pH
B)acid
C)base
D)Buffer

Unlock Deck
Unlock for access to all 67 flashcards in this deck.
Unlock Deck
k this deck


