Exam 2: Life's Chemical Bases
Exam 1: Invitation to Biology79 Questions
Exam 2: Life's Chemical Bases67 Questions
Exam 3: Molecules of Life87 Questions
Exam 4: Cell Structure106 Questions
Exam 5: Ground Rules of Metabolism69 Questions
Exam 6: Where it Starts—Photosynthesis69 Questions
Exam 7: How Cells Release Chemical Energy75 Questions
Exam 8: DNA Structure and Function61 Questions
Exam 9: From DNA to Protein64 Questions
Exam 10: Control of Gene Expression63 Questions
Exam 11: How Cells Reproduce77 Questions
Exam 12: Meiosis and Sexual Reproduction59 Questions
Exam 13: Observing Patterns in Inherited Traits95 Questions
Exam 14: Chromosomes and Human Inheritance76 Questions
Exam 15: Studying and Manipulating Genomes58 Questions
Exam 16: Evidence of Evolution55 Questions
Exam 17: Processes of Evolution74 Questions
Exam 18: Organizing Information about Species46 Questions
Exam 19: Life's Origin and Early Evolution60 Questions
Exam 20: Viruses, Bacteria, and Archaea56 Questions
Exam 21: Protists: The Simplest Eukaryotes61 Questions
Exam 22: The Land Plants66 Questions
Exam 23: Fungi52 Questions
Exam 24: Animal Evolution: The Invertebrates74 Questions
Exam 25: Animal Evolution: The Chordates71 Questions
Exam 26: Human Evolution51 Questions
Exam 27: Plant Tissues96 Questions
Exam 28: Plant Nutrition and Transport69 Questions
Exam 29: Life Cycles of Flowering Plants89 Questions
Exam 30: Communication Strategies in Plants72 Questions
Exam 31: Animal Tissues and Organ Systems79 Questions
Exam 32: Neural Control97 Questions
Exam 33: Sensory Perception69 Questions
Exam 34: Endocrine Control90 Questions
Exam 35: Structural Support and Movement72 Questions
Exam 36: Circulation58 Questions
Exam 37: Immunity73 Questions
Exam 38: Respiration78 Questions
Exam 39: Digestion and Nutrition98 Questions
Exam 40: Maintaining the Internal Environment73 Questions
Exam 41: Animal Reproductive System105 Questions
Exam 42: Animal Development72 Questions
Exam 43: Animal Behavior74 Questions
Exam 44: Population Ecology59 Questions
Exam 45: Community Ecology71 Questions
Exam 46: Ecosystems64 Questions
Exam 47: The Biosphere73 Questions
Exam 48: Human Impacts on the Biosphere67 Questions
Select questions type
How does the energy of an electron relate with the distance from the nucleus?
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
B
Figure 2.5A
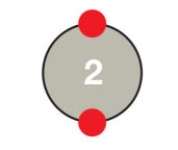 -Based on its outer shell,the atom in the accompanying figure would be characterized as ____.
-Based on its outer shell,the atom in the accompanying figure would be characterized as ____.
(Multiple Choice)
4.8/5  (29)
(29)
Classification. The various energy levels in an atom of magnesium (24Mg) have different numbers of electrons. Use the numbers below to answer the following questions.
-The number of electrons in the second energy level
(Multiple Choice)
4.9/5  (39)
(39)
Figure 2.5B
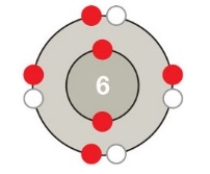 -Which atom is depicted in the accompanying figure?
-Which atom is depicted in the accompanying figure?
(Multiple Choice)
4.8/5  (37)
(37)
Classification. The following are types of chemical bonds. Answer the questions below by matching the descriptions with the most appropriate bond type.
-The bond that breaks when salts dissolve in water
(Multiple Choice)
4.9/5  (38)
(38)
An atom can get rid of vacancies by participating in a ___________.
(Multiple Choice)
4.9/5  (36)
(36)
The ability of a solution to resist changes in pH depends on its ____________________ capacity.
(Short Answer)
4.8/5  (35)
(35)
Classification. The following are important terms relating to acids and bases. Answer the questions below by matching the descriptions with the most appropriate word.
-Toothpaste
(Multiple Choice)
4.9/5  (33)
(33)
Classification. The following are important terms relating to acids and bases. Answer the questions below by matching the descriptions with the most appropriate word.
-Measure of H+ in a fluid
(Multiple Choice)
4.9/5  (36)
(36)
Figure 2.11B
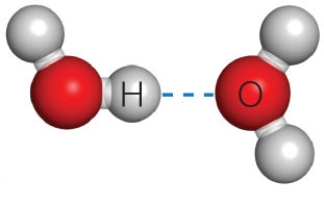 -The dashed line in the accompanying figure represents a(n)____.
-The dashed line in the accompanying figure represents a(n)____.
(Multiple Choice)
4.9/5  (31)
(31)
Tracers allow scientists to track a molecule through a biochemical process by replacing an atom in that molecule with its _________.
(Short Answer)
4.8/5  (33)
(33)
In the chemical shorthand,14C,the 14 represents the number of ____.
(Multiple Choice)
4.8/5  (32)
(32)
The predictable rate of ____________________ allows scientists to estimate the age of a rock or fossil by examining its isotope content.
(Short Answer)
4.9/5  (39)
(39)
A hydrogen bond is an attraction between a(n)____ hydrogen atom and another atom taking part in ____.
(Multiple Choice)
4.8/5  (32)
(32)
Classification. The following are important terms relating to acids and bases. Answer the questions below by matching the descriptions with the most appropriate word.
-Substance that releases,but does not accept,H+
(Multiple Choice)
4.9/5  (27)
(27)
Showing 1 - 20 of 67
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)