Deck 9: Models of Chemical Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/61
Play
Full screen (f)
Deck 9: Models of Chemical Bonding
1
For which of the following elements (in their normal, stable, forms) would it be correct to describe the bonding as involving "electron pooling"?
A) hydrogen
B) helium
C) sulfur
D) iodine
E) aluminum
A) hydrogen
B) helium
C) sulfur
D) iodine
E) aluminum
aluminum
2
In which of these substances are the atoms held together by metallic bonding?
A) CO2
B) Si
C) Br2
D) S8
E) Cr
A) CO2
B) Si
C) Br2
D) S8
E) Cr
Cr
3
Which of the following contains ionic bonding?
A) CO
B) SrF2
C) Al
D) OCl2
E) HCl
A) CO
B) SrF2
C) Al
D) OCl2
E) HCl
SrF2
4
Select the element whose Lewis symbol is correct.
A)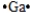
B)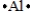
C)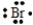
D)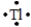
E)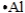
A)
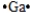
B)
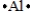
C)
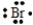
D)
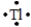
E)
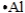

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
5
Select the compound with the highest lattice energy.
A) CaS(s)
B) BaO(s)
C) NaI(s)
D) LiBr(s)
E) MgO(s)
A) CaS(s)
B) BaO(s)
C) NaI(s)
D) LiBr(s)
E) MgO(s)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
6
Select the compound with the lowest (i.e., least negative) lattice energy.
A) CsBr(s)
B) NaCl(s)
C) SrO(s)
D) CaO(s)
E) KBr(s)
A) CsBr(s)
B) NaCl(s)
C) SrO(s)
D) CaO(s)
E) KBr(s)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
7
Select the correct formula for a compound formed from calcium and chlorine.
A) CaCl
B) CaCl2
C) Ca2Cl
D) Ca2Cl2
E) CaCl3
A) CaCl
B) CaCl2
C) Ca2Cl
D) Ca2Cl2
E) CaCl3

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
8
The diameter of a chloride ion is 362 pm, and the diameter of a potassium ion is 276 pm. What is the distance between the nuclei of adjacent chloride and potassium ions in solid potassium chloride?
A) 1276 pm
B) 638 pm
C) 319 pm
D) 181 pm
E) 138 pm
A) 1276 pm
B) 638 pm
C) 319 pm
D) 181 pm
E) 138 pm

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
9
Select the element whose Lewis symbol is correct.
A)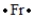
B)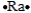
C)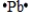
D)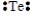
E)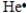
A)
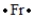
B)
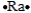
C)
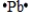
D)
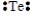
E)
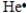

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
10
Calculate the lattice energy of magnesium sulfide from the data given below. Mg(s) → Mg(g) ΔH° = 148 kJ/mol
Mg(g) → Mg2+(g) + 2e- ΔH° = 2186 kJ/mol
S8(s) → 8S(g) ΔH° = 2232 kJ/mol
S(g) + 2e- → S2-(g) ΔH° = 450 kJ/mol
8Mg(s) + S8(s) → 8MgS(s) ΔH° = -2744 kJ/mol
Mg2+(g) + S2-(g) → MgS(s) ΔH°lattice = ?
A) -3406 kJ/mol
B) -2720. kJ/mol
C) 2720. kJ/mol
D) 3406 kJ/mol
E) None of these choices are correct.
Mg(g) → Mg2+(g) + 2e- ΔH° = 2186 kJ/mol
S8(s) → 8S(g) ΔH° = 2232 kJ/mol
S(g) + 2e- → S2-(g) ΔH° = 450 kJ/mol
8Mg(s) + S8(s) → 8MgS(s) ΔH° = -2744 kJ/mol
Mg2+(g) + S2-(g) → MgS(s) ΔH°lattice = ?
A) -3406 kJ/mol
B) -2720. kJ/mol
C) 2720. kJ/mol
D) 3406 kJ/mol
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is a covalent compound?
A) Na2O
B) CaCl2
C) Cl2O
D) CsCl
E) Al2O3
A) Na2O
B) CaCl2
C) Cl2O
D) CsCl
E) Al2O3

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
12
The lattice energy of MgCl2 is the energy change for which one of the following processes?
A) Mg(s) + Cl2(g) → MgCl2(s)
B) Mg(g) + 2Cl(g) → MgCl2(s)
C) Mg2+(s) + 2Cl-(g) → MgCl2(g)
D) Mg2+(g) + 2Cl-(g) → MgCl2(s)
E) MgCl2(aq) → MgCl2(s)
A) Mg(s) + Cl2(g) → MgCl2(s)
B) Mg(g) + 2Cl(g) → MgCl2(s)
C) Mg2+(s) + 2Cl-(g) → MgCl2(g)
D) Mg2+(g) + 2Cl-(g) → MgCl2(s)
E) MgCl2(aq) → MgCl2(s)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
13
The lattice energy for ionic crystals increases as the charge on the ions _____________ and the size of the ions __________________.
A) increases; increases
B) increases; decreases
C) decreases; increases
D) decreases; decreases
E) None of these choices are correct.
A) increases; increases
B) increases; decreases
C) decreases; increases
D) decreases; decreases
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
14
Select the correct formula for a compound formed from barium and nitrogen.
A) BaN
B) BaN2
C) Ba2N3
D) Ba2N
E) Ba3N2
A) BaN
B) BaN2
C) Ba2N3
D) Ba2N
E) Ba3N2

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
15
A Born-Haber cycle applied to the formation reaction of an ionic solid
A) is normally used to calculate ionization energy.
B) is normally used to calculate electron affinity.
C) is normally used to calculate bond energy.
D) is normally used to determine the overall enthalpy change.
E) is an application of Hess's Law.
A) is normally used to calculate ionization energy.
B) is normally used to calculate electron affinity.
C) is normally used to calculate bond energy.
D) is normally used to determine the overall enthalpy change.
E) is an application of Hess's Law.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is an ionic compound?
A) H2S
B) NH3
C) I2
D) KI
E) CCl4
A) H2S
B) NH3
C) I2
D) KI
E) CCl4

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
17
In which of these substances are the atoms held together by polar covalent bonding?
A) SrCl2
B) CsCl
C) ClF
D) TiF2
E) S8
A) SrCl2
B) CsCl
C) ClF
D) TiF2
E) S8

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
18
The lattice energy of CaF2 is the energy change for which one, if any, of the following processes?
A) Ca2+(s) + 2F-(g) → CaF2(g)
B) CaF2(g) → CaF2(s)
C) Ca(g) + 2F(g) → CaF2(s)
D) CaF2(aq) → CaF2(s)
E) None of these choices are correct.
A) Ca2+(s) + 2F-(g) → CaF2(g)
B) CaF2(g) → CaF2(s)
C) Ca(g) + 2F(g) → CaF2(s)
D) CaF2(aq) → CaF2(s)
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following contains covalent bonds?
A) BaO
B) IBr
C) Mg
D) LiBr
E) Cu
A) BaO
B) IBr
C) Mg
D) LiBr
E) Cu

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
20
Analysis of an unknown substance showed that it has a high boiling point and is brittle. It is an insulator as a solid but conducts electricity when melted. Which of the following substances would have those characteristics?
A) HCl
B) Al
C) KBr
D) SiF4
E) I2
A) HCl
B) Al
C) KBr
D) SiF4
E) I2

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
21
Using the bond energies provided below, calculate ΔH° for the reaction CH4(g) + 4Cl2(g) → CCl4(g) + 4HCl(g)
Bond energies: C-H = 413 kJ/mol, Cl-Cl = 243 kJ/mol, C-Cl = 339 kJ/mol, H-Cl = 427 kJ/mol
A) 1422 kJ
B) 440 kJ
C) 110 kJ
D) -110 kJ
E) - 440 kJ
Bond energies: C-H = 413 kJ/mol, Cl-Cl = 243 kJ/mol, C-Cl = 339 kJ/mol, H-Cl = 427 kJ/mol
A) 1422 kJ
B) 440 kJ
C) 110 kJ
D) -110 kJ
E) - 440 kJ

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
22
Arrange calcium, rubidium, sulfur, and arsenic in order of decreasing electronegativity.
A) S > As > Rb > Ca
B) S > As > Ca > Rb
C) As > S > Rb > Ca
D) As > S > Ca > Rb
E) None of these choices are correct.
A) S > As > Rb > Ca
B) S > As > Ca > Rb
C) As > S > Rb > Ca
D) As > S > Ca > Rb
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
23
Which one of the following properties is least characteristic of substances composed of small, covalently-bonded molecules?
A) low melting point
B) low boiling point
C) weak bonds
D) poor electrical conductor when solid
E) poor electrical conductor when molten
A) low melting point
B) low boiling point
C) weak bonds
D) poor electrical conductor when solid
E) poor electrical conductor when molten

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
24
Arrange aluminum, boron, nitrogen, and phosphorous in order of increasing electronegativity.
A) AlB) BC) AlD) NE) None of these choices are correct.
A) Al

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
25
Combustion of a fat will release more energy than combustion of an equal mass of carbohydrate because
A) fats contain more bonds to oxygen than carbohydrates.
B) fats contain fewer bonds to oxygen than carbohydrates.
C) the total energy of the carbon-carbon and carbon-hydrogen bonds in fats is greater than the energy content of the carbon-oxygen and oxygen-hydrogen bonds in the reaction products (carbon dioxide and water).
D) the total energy of the carbon-carbon and carbon-hydrogen bonds in fats is greater than the energy content of the bonds in carbohydrates.
E) fats have higher molar masses than carbohydrates.
A) fats contain more bonds to oxygen than carbohydrates.
B) fats contain fewer bonds to oxygen than carbohydrates.
C) the total energy of the carbon-carbon and carbon-hydrogen bonds in fats is greater than the energy content of the carbon-oxygen and oxygen-hydrogen bonds in the reaction products (carbon dioxide and water).
D) the total energy of the carbon-carbon and carbon-hydrogen bonds in fats is greater than the energy content of the bonds in carbohydrates.
E) fats have higher molar masses than carbohydrates.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
26
Arrange the following bonds in order of increasing bond strength.
A) C-I < C-Br < C-Cl < C-F
B) C-F < C-Cl < C-Br < C-I
C) C-Br < C-I < C-Cl < C-F
D) C-I < C-Br < C-F< C-Cl
E) None of these choices are correct.
A) C-I < C-Br < C-Cl < C-F
B) C-F < C-Cl < C-Br < C-I
C) C-Br < C-I < C-Cl < C-F
D) C-I < C-Br < C-F< C-Cl
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
27
Arrange oxygen, sulfur, calcium, rubidium, and potassium in order of decreasing electronegativity.
A) O > S > Ca > K > Rb
B) O > S > Ca > Rb > K
C) O > S > Rb > K > Ca
D) O > S > Rb > Ca > K
E) None of these choices are correct.
A) O > S > Ca > K > Rb
B) O > S > Ca > Rb > K
C) O > S > Rb > K > Ca
D) O > S > Rb > Ca > K
E) None of these choices are correct.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
28
Select the strongest bond in the following group.
A) C-S
B) C-O
C) C=C
D) C≡N
E) C-F
A) C-S
B) C-O
C) C=C
D) C≡N
E) C-F

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
29
Which one of the following properties is least characteristic of typical ionic compounds?
A) high melting point
B) high boiling point
C) brittleness
D) poor electrical conductor when solid
E) poor electrical conductor when molten
A) high melting point
B) high boiling point
C) brittleness
D) poor electrical conductor when solid
E) poor electrical conductor when molten

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
30
Electronegativity is a measure of
A) the energy needed to remove an electron from an atom.
B) the energy released when an electron is added to an atom.
C) the magnitude of the negative charge on an electron.
D) the attraction by an atom for electrons in a chemical bond.
E) the magnitude of the negative charge on a molecule.
A) the energy needed to remove an electron from an atom.
B) the energy released when an electron is added to an atom.
C) the magnitude of the negative charge on an electron.
D) the attraction by an atom for electrons in a chemical bond.
E) the magnitude of the negative charge on a molecule.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
31
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon-oxygen double bond. Calculate the enthalpy of reaction using the bond energies given. 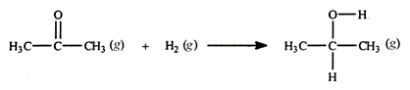 Bond: C=O H-H C-H O-H C-C C-O Bond energy (kJ/mol): 745 432 413 467 347 358
Bond: C=O H-H C-H O-H C-C C-O Bond energy (kJ/mol): 745 432 413 467 347 358
A) -484 kJ
B) -366 kJ
C) -61 kJ
D) +61 kJ
E) +366 kJ
 Bond: C=O H-H C-H O-H C-C C-O Bond energy (kJ/mol): 745 432 413 467 347 358
Bond: C=O H-H C-H O-H C-C C-O Bond energy (kJ/mol): 745 432 413 467 347 358A) -484 kJ
B) -366 kJ
C) -61 kJ
D) +61 kJ
E) +366 kJ

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
32
When one mole of each of the following liquids is burned, which will produce the most heat energy?
A) C6H14
B) C5H12
C) C6H14O
D) C6H12O
E) C6H10O3
A) C6H14
B) C5H12
C) C6H14O
D) C6H12O
E) C6H10O3

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following elements is the most electronegative?
A) S
B) Ru
C) Si
D) Te
E) Cs
A) S
B) Ru
C) Si
D) Te
E) Cs

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following elements is the least electronegative?
A) Si
B) Se
C) S
D) Sc
E) Sr
A) Si
B) Se
C) S
D) Sc
E) Sr

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
35
Nitrogen and hydrogen combine to form ammonia in the Haber process. Calculate (in kJ) the standard enthalpy change ΔH° for the reaction written below, using the bond energies given. N2(g) + 3H2(g) → 2NH3(g)
Bond: N≡N H-H N-H
Bond energy(kJ/mol): 945 432 391
A) -969 kJ
B) -204 kJ
C) -105 kJ
D) 204 kJ
E) 595 kJ
Bond: N≡N H-H N-H
Bond energy(kJ/mol): 945 432 391
A) -969 kJ
B) -204 kJ
C) -105 kJ
D) 204 kJ
E) 595 kJ

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
36
Based on electronegativity trends in the periodic table, predict which of the following compounds will have the greatest % ionic character in its bonds.
A) H2O
B) LiI
C) CaO
D) RbF
E) HCl
A) H2O
B) LiI
C) CaO
D) RbF
E) HCl

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
37
Hydrogenation of double and triple bonds is an important industrial process. Calculate (in kJ) the standard enthalpy change ΔH° for the hydrogenation of ethyne (acetylene) to ethane. H-C≡C-H(g) + 2H2(g) → H3C-CH3(g)
Bond: C-C C≡C C-H H-H
Bond energy (kJ/mol): 347 839 413 432
A) -296 kJ
B) -51 kJ
C) 51 kJ
D) 296 kJ
E) 381 kJ
Bond: C-C C≡C C-H H-H
Bond energy (kJ/mol): 347 839 413 432
A) -296 kJ
B) -51 kJ
C) 51 kJ
D) 296 kJ
E) 381 kJ

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following elements is the most electronegative?
A) Ne
B) Rb
C) P
D) I
E) Cl
A) Ne
B) Rb
C) P
D) I
E) Cl

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
39
When two atoms form a covalently-bonded diatomic molecule, the distance between the nuclei at which the potential energy is at a minimum is called
A) the bond energy.
B) the bond length.
C) the molecular diameter.
D) the covalent radius.
E) the covalent diameter.
A) the bond energy.
B) the bond length.
C) the molecular diameter.
D) the covalent radius.
E) the covalent diameter.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
40
Quartz (SiO2) is a solid with a melting point of 1550 °C. The bonding in quartz is best described as
A) lattice energy.
B) network attractions.
C) ionic bonding.
D) covalent bonding.
E) metallic bonding.
A) lattice energy.
B) network attractions.
C) ionic bonding.
D) covalent bonding.
E) metallic bonding.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
41
No real bonds are 100% ionic in character.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
42
In covalent bond formation, the potential energy reaches a maximum when the internuclear distance is equal to the bond length.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following period 3 chlorides would be expected to have the highest melting point?
A) MgCl2
B) AlCl3
C) SiCl4
D) PCl3
E) SCl2
A) MgCl2
B) AlCl3
C) SiCl4
D) PCl3
E) SCl2

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
44
Covalently bonded substances do not necessarily exist as separate molecules.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
45
Select the most polar bond amongst the following.
A) C-O
B) Si-F
C) Cl-F
D) C-F
E) C-I
A) C-O
B) Si-F
C) Cl-F
D) C-F
E) C-I

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following compounds displays the greatest ionic character in its bonds?
A) NO2
B) CO2
C) H2O
D) HF
E) NH3
A) NO2
B) CO2
C) H2O
D) HF
E) NH3

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
47
Bond energy increases as bond order increases, for bonding between a given pair of atoms.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
48
A single covalent bond consists of a single delocalized electron pair.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
49
The electrostatic energy of two charged particles is inversely proportional to the square of the distance between them.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
50
Analysis of an unknown substance showed that it has a moderate melting point and is a good conductor of heat and electricity in the solid phase. Which of the following substances would have those characteristics?
A) NaCl
B) Si
C) CCl4
D) I2
E) Ga
A) NaCl
B) Si
C) CCl4
D) I2
E) Ga

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
51
The melting points of metals are only moderately high because
A) metallic bonding is weak.
B) metals have fewer bonding electrons than nonmetals.
C) metals also have relatively low boiling points.
D) the melting process does not break the metallic bonds.
E) metals prefer to be bonded to nonmetals.
A) metallic bonding is weak.
B) metals have fewer bonding electrons than nonmetals.
C) metals also have relatively low boiling points.
D) the melting process does not break the metallic bonds.
E) metals prefer to be bonded to nonmetals.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
52
The majority of elements are good electrical conductors when in solid form.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
53
The lattice energy is the energy released when separated ions in the gas phase combine to form ionic molecules in the gas phase.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
54
The stronger the bonds in a fuel, the more energy it will yield.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
55
Electronegativities on Pauling's scale are calculated from ionization energies and electron affinities.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
56
As a measure of the strength of metallic bonding, the boiling point of a metal is a better indicator than its melting point.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
57
The lattice energy of large ions is greater in magnitude than that of small ions of the same charge.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
58
The more C-O and O-H bonds there are in a substance, the greater will be the amount of heat released when a fixed mass of the substance is burned.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
59
Which one of the following properties is least characteristic of typical metals?
A) moderately high melting point
B) high boiling point
C) brittleness
D) good electrical conductor when solid
E) good electrical conductor when molten
A) moderately high melting point
B) high boiling point
C) brittleness
D) good electrical conductor when solid
E) good electrical conductor when molten

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
60
The electrostatic energy of two charged particles is inversely proportional to the distance between them.

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck
61
In which of the following processes does the enthalpy change (ΔH) directly represent the magnitude of the lattice energy of KCl(s)?
A) Cl2(g) + 2K(s) → 2KC (s)
B) KCl(s) → K+(aq) + Cl- (aq)
C) KCl(s) → K+(g) + Cl-(g)
D) KCl(s) → K(s) + Cl-(g)
E) KCl(s) → K(s) + Cl(g)
A) Cl2(g) + 2K(s) → 2KC (s)
B) KCl(s) → K+(aq) + Cl- (aq)
C) KCl(s) → K+(g) + Cl-(g)
D) KCl(s) → K(s) + Cl-(g)
E) KCl(s) → K(s) + Cl(g)

Unlock Deck
Unlock for access to all 61 flashcards in this deck.
Unlock Deck
k this deck


