Exam 9: Models of Chemical Bonding
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Which of the following period 3 chlorides would be expected to have the highest melting point?
Free
(Multiple Choice)
4.7/5  (36)
(36)
Correct Answer:
A
Select the compound with the lowest (i.e., least negative) lattice energy.
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
A
Select the correct formula for a compound formed from calcium and chlorine.
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
B
The melting points of metals are only moderately high because
(Multiple Choice)
4.8/5  (35)
(35)
In which of these substances are the atoms held together by metallic bonding?
(Multiple Choice)
4.8/5  (35)
(35)
Nitrogen and hydrogen combine to form ammonia in the Haber process. Calculate (in kJ) the standard enthalpy change ΔH° for the reaction written below, using the bond energies given. N2(g) + 3H2(g) → 2NH3(g)
Bond: N≡N H-H N-H
Bond energy(kJ/mol): 945 432 391
(Multiple Choice)
4.8/5  (23)
(23)
The more C-O and O-H bonds there are in a substance, the greater will be the amount of heat released when a fixed mass of the substance is burned.
(True/False)
4.9/5  (32)
(32)
Which of the following elements is the most electronegative?
(Multiple Choice)
4.9/5  (29)
(29)
Select the correct formula for a compound formed from barium and nitrogen.
(Multiple Choice)
4.9/5  (39)
(39)
For which of the following elements (in their normal, stable, forms) would it be correct to describe the bonding as involving "electron pooling"?
(Multiple Choice)
4.7/5  (36)
(36)
The lattice energy for ionic crystals increases as the charge on the ions _____________ and the size of the ions __________________.
(Multiple Choice)
4.7/5  (35)
(35)
The lattice energy of large ions is greater in magnitude than that of small ions of the same charge.
(True/False)
4.8/5  (34)
(34)
In covalent bond formation, the potential energy reaches a maximum when the internuclear distance is equal to the bond length.
(True/False)
4.9/5  (26)
(26)
Which of the following elements is the least electronegative?
(Multiple Choice)
4.8/5  (41)
(41)
The majority of elements are good electrical conductors when in solid form.
(True/False)
4.8/5  (29)
(29)
Acetone can be easily converted to isopropyl alcohol by addition of hydrogen to the carbon-oxygen double bond. Calculate the enthalpy of reaction using the bond energies given.
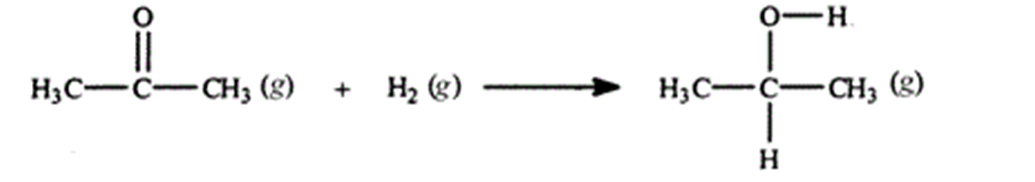
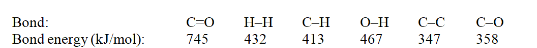
(Multiple Choice)
4.9/5  (39)
(39)
Arrange the following bonds in order of increasing bond strength.
(Multiple Choice)
4.7/5  (42)
(42)
Which one of the following properties is least characteristic of typical ionic compounds?
(Multiple Choice)
4.7/5  (24)
(24)
Calculate the lattice energy of magnesium sulfide from the data given below. Mg(s) → Mg(g) ΔH° = 148 kJ/mol
Mg(g) → Mg2+(g) + 2e- ΔH° = 2186 kJ/mol
S8(s) → 8S(g) ΔH° = 2232 kJ/mol
S(g) + 2e- → S2-(g) ΔH° = 450 kJ/mol
8Mg(s) + S8(s) → 8MgS(s) ΔH° = -2744 kJ/mol
Mg2+(g) + S2-(g) → MgS(s) ΔH°lattice = ?
(Multiple Choice)
4.8/5  (33)
(33)
Showing 1 - 20 of 61
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)