Deck 5: Ground Rules of Metabolism
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/58
Play
Full screen (f)
Deck 5: Ground Rules of Metabolism
1
An enzyme found inside a human cell ____.
A)can catalyze many different reactions by binding to various substrates
B)helps bring reactants to the transition state
C)can catalyze endergonic reactions only
D)rarely requires cofactors
E)works optimally at temperatures below 37oC
A)can catalyze many different reactions by binding to various substrates
B)helps bring reactants to the transition state
C)can catalyze endergonic reactions only
D)rarely requires cofactors
E)works optimally at temperatures below 37oC
B
2
The enzyme hexokinase catalyzes the addition of a phosphate group (taken from ATP) to a glucose molecule. Magnesium (Mg++), which is required for hexokinase to function properly, interacts with the ATP to facilitate binding to the hexokinase active site. Magnesium is therefore a(n) ____.
A)product
B)reactant
C)cofactor
D)substrate
E)enzyme
A)product
B)reactant
C)cofactor
D)substrate
E)enzyme
C
3
Which biological molecule(s) may show enzymatic activity?
A)lipids only
B)proteins only
C)RNA only
D)RNA and proteins
E)proteins and carbohydrates
A)lipids only
B)proteins only
C)RNA only
D)RNA and proteins
E)proteins and carbohydrates
D
4
All of these substances assist enzymes during the catalysis of biochemical reactions. Which one is a cofactor (that is, not a coenzyme)?
A)ATP
B)heme
C)vitamin B7 (biotin)
D)flavine adenine dinucleotide (FAD)
E)nicotine adenine dinucleotide phosphate (NADP)
A)ATP
B)heme
C)vitamin B7 (biotin)
D)flavine adenine dinucleotide (FAD)
E)nicotine adenine dinucleotide phosphate (NADP)

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
5
Some enzymes require inorganic or organic molecules for proper function. These molecules are known as ____.
A)coenzymes only
B)cofactors only
C)antioxidants only
D)coenzymes and cofactors
E)coenzymes, cofactors, and antioxidants
A)coenzymes only
B)cofactors only
C)antioxidants only
D)coenzymes and cofactors
E)coenzymes, cofactors, and antioxidants

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
6
What is occurring when a transport protein uses energy to pump a solute across a cell membrane against its concentration gradient?
A)osmosis
B)passive diffusion
C)facilitated diffusion
D)phagocytosis
E)active transport
A)osmosis
B)passive diffusion
C)facilitated diffusion
D)phagocytosis
E)active transport

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
7
White blood cells use ____ to remove foreign particles from the blood.
A)simple diffusion
B)bulk flow
C)osmosis
D)phagocytosis
E)facilitated diffusion
A)simple diffusion
B)bulk flow
C)osmosis
D)phagocytosis
E)facilitated diffusion

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
8
The analogy of a key fitting into a lock is descriptive of the ____.
A)random collision between substrate molecules
B)binding of coenzymes to enzymes
C)binding of substrate to the enzyme's active site
D)regeneration of ATP from ADP
E)stepwise cascade of electrons in the oxidation-reduction reactions
A)random collision between substrate molecules
B)binding of coenzymes to enzymes
C)binding of substrate to the enzyme's active site
D)regeneration of ATP from ADP
E)stepwise cascade of electrons in the oxidation-reduction reactions

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
9
During enzyme-catalyzed reactions, the substrate is also known as the ____.
A)end product
B)byproduct
C)enzyme
D)reactant
E)cofactor
A)end product
B)byproduct
C)enzyme
D)reactant
E)cofactor

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
10
In a cyclic metabolic pathway, the ____.
A)product inhibits the activity of the first enzyme in the pathway
B)last step regenerates the enzyme that catalyzes the first step in the pathway
C)product inhibits the activity of the enzyme that catalyzes the last step in the pathway
D)last step regenerates the reactant for the first step in the pathway
E)regulatory molecules bind to regions of enzymes other than the active site
A)product inhibits the activity of the first enzyme in the pathway
B)last step regenerates the enzyme that catalyzes the first step in the pathway
C)product inhibits the activity of the enzyme that catalyzes the last step in the pathway
D)last step regenerates the reactant for the first step in the pathway
E)regulatory molecules bind to regions of enzymes other than the active site

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
11
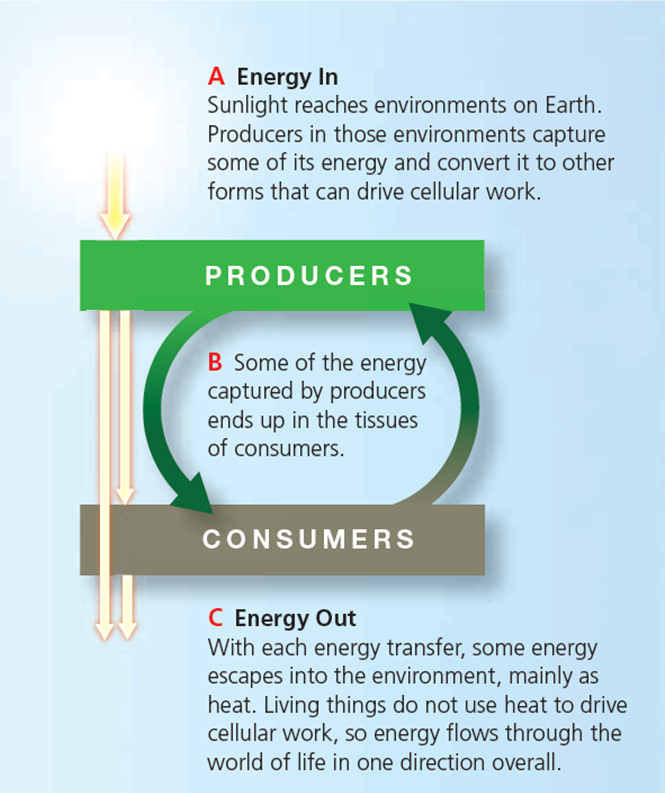
-The accompanying figure illustrates the ____ biological systems.
A)destruction of energy in
B)dispersion of chemical bond energy throughout
C)decrease in entropy in
D)one-way flow of energy through
E)bidirectional flow of energy through

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
12
A single-celled freshwater organism, such as a protistan, is transferred to salt water. What is likely to happen?
A)The cell will burst.
B)Salt will be pumped out of the cell.
C)The cell will shrink.
D)Enzymes will flow out of the cell.
E)Water will move into the cell.
A)The cell will burst.
B)Salt will be pumped out of the cell.
C)The cell will shrink.
D)Enzymes will flow out of the cell.
E)Water will move into the cell.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
13
The rate of diffusion through a semipermeable membrane is slowest when ____.
A)molecules are small
B)there is a concentration gradient
C)there is a difference in charge
D)there is high pressure
E)temperatures are low
A)molecules are small
B)there is a concentration gradient
C)there is a difference in charge
D)there is high pressure
E)temperatures are low

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
14
Suppose that it takes three pounds of feed to generate one pound of chicken meat. What happens to the other two pounds of feed?
A)Energy is lost in substances that are indigestible only.
B)Energy is lost as heat only.
C)Energy is lost in substances that are indigestible and as heat.
D)Energy is lost to maintain constant entropy in the system.
E)Energy is destroyed during the process of energy conversion.
A)Energy is lost in substances that are indigestible only.
B)Energy is lost as heat only.
C)Energy is lost in substances that are indigestible and as heat.
D)Energy is lost to maintain constant entropy in the system.
E)Energy is destroyed during the process of energy conversion.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
15
A teacher at the front of a classroom sprays a mist of perfume into the air. After a few minutes, the students in the back of the room are able to smell the perfume. This is an example of ____.
A)increasing entropy only
B)diffusion only
C)decreasing entropy only
D)increasing entropy and diffusion
E)decreasing entropy and diffusion
A)increasing entropy only
B)diffusion only
C)decreasing entropy only
D)increasing entropy and diffusion
E)decreasing entropy and diffusion

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
16
During endergonic reactions, reactants with ____ free energy are converted to molecules with ____ free energy; therefore, they require a net input of energy in order to proceed.
A)higher; lower
B)lower; higher
C)zero; lower
D)higher; zero
E)potential; kinetic
A)higher; lower
B)lower; higher
C)zero; lower
D)higher; zero
E)potential; kinetic

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
17
Activation energy is the energy required to bring the bonds in reactant molecules to ____.
A)the active site
B)their strongest conformation
C)a reducing agent
D)their breaking point
E)an oxidizing agent
A)the active site
B)their strongest conformation
C)a reducing agent
D)their breaking point
E)an oxidizing agent

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
18
Adding heat to molecules boosts their free energy. The greater the free energy of reactants, the closer they are to ____.
A)feedback inhibition
B)an exergonic reaction
C)an induced fit with an enzyme
D)the transition state
E)cofactor interaction
A)feedback inhibition
B)an exergonic reaction
C)an induced fit with an enzyme
D)the transition state
E)cofactor interaction

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
19

-In the metabolic pathway of ethanol breakdown, as shown in the accompanying figure, ____.?
A)NAD+ is a coenzyme for both the ADH and ALDH enzymes
B)acetate is a toxic metabolite, leading to the effects of a hangover
C)acetaldehyde is a harmless metabolite which is excreted by the body
D)a lack of ALDH enzyme will lessen the effects of a hangover
E)ethanol is converted to acetaldehyde, a less toxic intermediate

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
20
Which reaction is endergonic?
A)protein synthesis
B)digestion
C)fire
D)aerobic respiration
E)movement
A)protein synthesis
B)digestion
C)fire
D)aerobic respiration
E)movement

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
21
Enzymes increase the rate of a given reaction by lowering ____ energy.
A)kinetic
B)activation
C)thermal
D)potential
E)chemical bond
A)kinetic
B)activation
C)thermal
D)potential
E)chemical bond

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
22
Pepsin and trypsin are digestive enzymes. In the stomach, normal pH is 2, while in the small intestine, normal pH is about 7.5. Based on this information and the accompanying figure, ____.
A)trypsin functions optimally in the small intestine
B)trypsin functions optimally in the stomach
C)both trypsin and pepsin function optimally in the stomach
D)both trypsin and pepsin function optimally in the small intestine
E)both pepsin and trypsin function equally well in the stomach and the small intestine
A)trypsin functions optimally in the small intestine
B)trypsin functions optimally in the stomach
C)both trypsin and pepsin function optimally in the stomach
D)both trypsin and pepsin function optimally in the small intestine
E)both pepsin and trypsin function equally well in the stomach and the small intestine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
23
In the formation of ATP from ADP and Pi, ADP is ____.
A)reduced
B)oxidized
C)phosphorylated
D)denatured
E)inactivated
A)reduced
B)oxidized
C)phosphorylated
D)denatured
E)inactivated

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
24
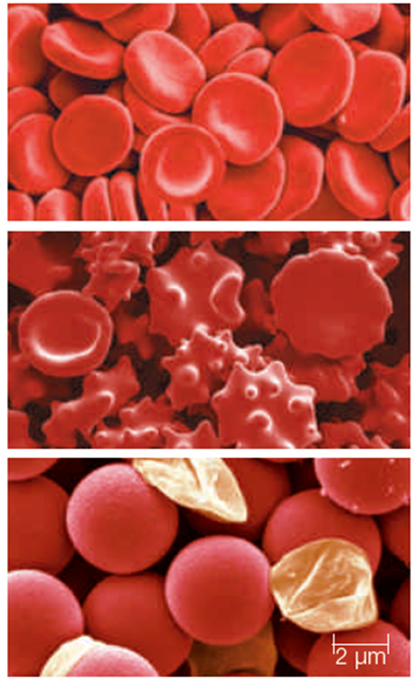
-The red blood cells shown in the middle micrograph of the accompanying figure are immersed in a(n) ____ solution.
A)hypotonic
B)isotonic
C)hypertonic
D)enzymatic
E)turgor

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
25
Wilting of a plant occurs when ____.
A)the plant cell walls weaken
B)osmotic pressure is reached
C)there is turgor
D)soil water becomes hypotonic
E)soil water becomes hypertonic
A)the plant cell walls weaken
B)osmotic pressure is reached
C)there is turgor
D)soil water becomes hypotonic
E)soil water becomes hypertonic

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
26
A car uses the energy stored in the organic molecules of gasoline to propel the vehicle forward. In this scenario, the ____ energy in the gasoline is converted to ____ energy of motion, illustrating the ____ law of thermodynamics.
A)potential; chemical bond; second
B)chemical; activation; second
C)kinetic; potential; first
D)potential; kinetic; first
E)activation; kinetic; second
A)potential; chemical bond; second
B)chemical; activation; second
C)kinetic; potential; first
D)potential; kinetic; first
E)activation; kinetic; second

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
27
A glucose transporter changes shape when it binds to a molecule of glucose. The shape change moves glucose down its concentration gradient to the opposite side of the membrane, where it detaches from the transport protein. What does this illustrate?
A)facilitated diffusion
B)endocytosis
C)active transport
D)exocytosis
E)osmosis
A)facilitated diffusion
B)endocytosis
C)active transport
D)exocytosis
E)osmosis

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
28
A passive process that requires a protein for the movement of a solute across a membrane is known as ____.
A)active transport
B)endocytosis
C)bulk flow
D)facilitated diffusion
E)osmosis
A)active transport
B)endocytosis
C)bulk flow
D)facilitated diffusion
E)osmosis

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
29
ATP contains ____.
A)adenine
B)cytosine
C)uracil
D)thymine
E)guanine
A)adenine
B)cytosine
C)uracil
D)thymine
E)guanine

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
30
The removal of electrons from a compound is known as ____.
A)dehydration
B)oxidation
C)reduction
D)phosphorylation
E)allosteric regulation
A)dehydration
B)oxidation
C)reduction
D)phosphorylation
E)allosteric regulation

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
31
The process by which an enzyme allows a reaction to run much faster than it would on its own is known as ____.
A)a redox reaction
B)allosteric regulation
C)induced fit
D)catalysis
E)phosphorylation
A)a redox reaction
B)allosteric regulation
C)induced fit
D)catalysis
E)phosphorylation

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
32
Which type of molecular movement is a passive process?
A)sodium-potassium pump
B)endocytosis
C)exocytosis
D)diffusion
E)active transport
A)sodium-potassium pump
B)endocytosis
C)exocytosis
D)diffusion
E)active transport

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
33
A car uses the energy stored in the organic molecules of gasoline to propel the vehicle forward. However, not all the energy contained in the fuel molecules is used for propulsion; instead, some is lost as heat. In this scenario, entropy is ____, illustrating the ____ law of thermodynamics.
A)increasing; second
B)decreasing; second
C)increasing; first
D)decreasing; second
E)staying the same; second
A)increasing; second
B)decreasing; second
C)increasing; first
D)decreasing; second
E)staying the same; second

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
34
Movement of a molecule against its concentration gradient always requires ____.
A)cofactors
B)electron transfer chains
C)oxidation
D)energy
E)reduction
A)cofactors
B)electron transfer chains
C)oxidation
D)energy
E)reduction

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
35
A cell placed in a(n) ____ solution will ____.
A)isotonic; swell
B)hypotonic; swell
C)hypotonic; shrink
D)hypertonic; remain the same size
E)hypertonic; swell
A)isotonic; swell
B)hypotonic; swell
C)hypotonic; shrink
D)hypertonic; remain the same size
E)hypertonic; swell

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
36
A(n) ____ is an organized series of reaction steps in which membrane-bound arrays of enzymes and other molecules give up and accept electrons in turn.
A)cyclic metabolic pathway
B)endergonic reaction
C)electron transfer chain
D)concentration gradient
E)passive transport
A)cyclic metabolic pathway
B)endergonic reaction
C)electron transfer chain
D)concentration gradient
E)passive transport

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
37
An enzyme ____.
A)is altered during the course of a reaction
B)becomes part of the product
C)becomes part of the reactants
D)is not altered during the course of a reaction
E)cannot be reused after catalyzing one reaction
A)is altered during the course of a reaction
B)becomes part of the product
C)becomes part of the reactants
D)is not altered during the course of a reaction
E)cannot be reused after catalyzing one reaction

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
38
Pressure that a fluid exerts against its container is called ____.
A)hypertonic
B)a pressure gradient
C)osmosis
D)turgor
E)isotonic
A)hypertonic
B)a pressure gradient
C)osmosis
D)turgor
E)isotonic

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
39
Which condition(s) increase the rate of diffusion through a semipermeable membrane?
I.Steep concentration gradients
II.High temperatures
III.Large molecules
A)I only
B)II only
C)I and II
D)II and III
E)I, II, and III
I.Steep concentration gradients
II.High temperatures
III.Large molecules
A)I only
B)II only
C)I and II
D)II and III
E)I, II, and III

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
40
Both endergonic and exergonic reactions require an input of ____ to begin.
A)organic molecules
B)initiation energy
C)activation energy
D)water
E)oxygen
A)organic molecules
B)initiation energy
C)activation energy
D)water
E)oxygen

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
41
A molecule that gives up an electron becomes ____.
A)ionized only
B)oxidized only
C)reduced only
D)both ionized and oxidized
E)both ionized and reduced
A)ionized only
B)oxidized only
C)reduced only
D)both ionized and oxidized
E)both ionized and reduced

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
42
Glucose enters a cell via a glucose transporter. What prevents glucose molecules from moving back through the transporter and leaving the cell?
A)phosphorylation
B)charge differences
C)closed transporters
D)gated transporters
E)concentration gradient
A)phosphorylation
B)charge differences
C)closed transporters
D)gated transporters
E)concentration gradient

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
43
The first law of thermodynamics states that ____.
A)one form of energy cannot be converted into another
B)entropy is increasing in the universe
C)energy can be neither created nor destroyed
D)energy cannot be converted into matter
E)energy is the capacity to do work
A)one form of energy cannot be converted into another
B)entropy is increasing in the universe
C)energy can be neither created nor destroyed
D)energy cannot be converted into matter
E)energy is the capacity to do work

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
44
ATP acts as a(n) ____ in almost all metabolic pathways.
A)coenzyme
B)feedback regulator
C)catalyst
D)allosteric regulator
E)enzyme
A)coenzyme
B)feedback regulator
C)catalyst
D)allosteric regulator
E)enzyme

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
45
Match the transport mechanism to the correct description.
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
the movement of water molecules across a semipermeable membrane
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
the movement of water molecules across a semipermeable membrane

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
46
Match the transport mechanism to the correct description.
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
general term that describes the movement of any substance from areas of higher concentration to areas of lower concentration
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
general term that describes the movement of any substance from areas of higher concentration to areas of lower concentration

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
47
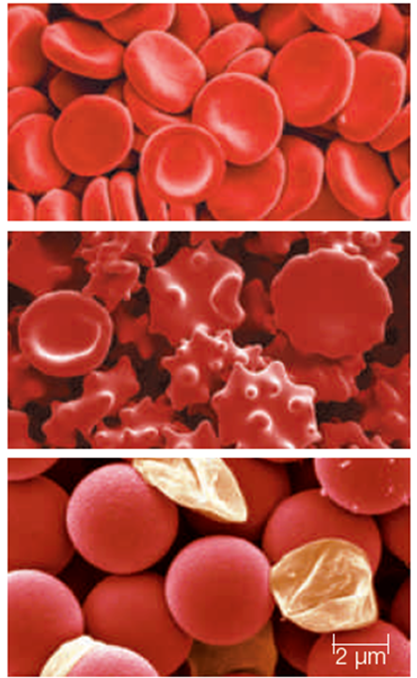
-The red blood cells shown in the top micrograph of the accompanying figure are immersed in a(n) ____ solution.
A)hypotonic
B)isotonic
C)hypertonic
D)enzymatic
E)turgor

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
48
Methane (CH4) is a natural gas that is often used as a fuel. When it burns in the presence of oxygen, carbon dioxide and water are produced. Replace the "X" in the following reaction of methane combustion to balance the equation. CH4 + 2O2 → CO2 + XH2O
A)lCH4 + 2O2 → CO2 + 0H2O
B)CH4 + 2O2 → CO2 + 1H2O
C)CH4 + 2O2 → CO2 + 2H2O
D)CH4 + 2O2 → CO2 + 32O
E)CH4 + 2O2 → CO2 + 4H2O
A)lCH4 + 2O2 → CO2 + 0H2O
B)CH4 + 2O2 → CO2 + 1H2O
C)CH4 + 2O2 → CO2 + 2H2O
D)CH4 + 2O2 → CO2 + 32O
E)CH4 + 2O2 → CO2 + 4H2O

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
49
Match the transport mechanism to the correct description.
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
the process by which a protein assists in passive transport
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
the process by which a protein assists in passive transport

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
50
The enzyme aspartate transcarbamoylase (ATCase) catalyzes the first step in the pyrimidine synthesis pathway. When cytosine and uracil nucleotides are present in excess, they bind to sites outside of the active site to inhibit ATCase. This scenario illustrates ____.
A)allosteric regulation only
B)feedback inhibition only
C)a redox reaction
D)allosteric regulation and feedback inhibition
E)feedback inhibition and a cyclic pathway
A)allosteric regulation only
B)feedback inhibition only
C)a redox reaction
D)allosteric regulation and feedback inhibition
E)feedback inhibition and a cyclic pathway

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
51
When ATP releases its energy, ____ is formed.
A)AMP only
B)ADP only
C)Pi only
D)both AMP and Pi
E)both ADP and Pi
A)AMP only
B)ADP only
C)Pi only
D)both AMP and Pi
E)both ADP and Pi

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
52
Allosteric regulation controls reaction rate by ____.
A)producing excess substrates
B)binding regulatory molecules at a site other than the active site
C)changing in the temperature of the system
D)binding regulatory molecules to the active site
E)alternately oxidizing and reducing the substrate
A)producing excess substrates
B)binding regulatory molecules at a site other than the active site
C)changing in the temperature of the system
D)binding regulatory molecules to the active site
E)alternately oxidizing and reducing the substrate

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
53
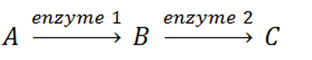
-If the accompanying metabolic pathway is regulated by feedback inhibition, ____ inhibits the activity of ____.?
A)C; enzyme 1
B)enzyme 1; B
C)enzyme 2; enzyme 1
D)enzyme 1; enzyme 2
E)?B; A

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
54
Match the transport mechanism to the correct description.
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
general term that describes the movement of molecules against a concentration gradient
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
general term that describes the movement of molecules against a concentration gradient

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
55
Match the transport mechanism to the correct description.
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
the condition in which water stops diffusing into the cytoplasm of a plant cell when enough pressure has built up inside it
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
the condition in which water stops diffusing into the cytoplasm of a plant cell when enough pressure has built up inside it

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
56
How does a high environmental pH affect an enzyme's activity?
A)High pH decreases the activity of all enzymes.
B)High pH increases the activity of all enzymes.
C)High pH changes the shape of all enzymes.
D)High pH does not affect the enzyme, but does lower the availability of substrate.
E)Some enzymes work well at a high pH, some work well at low pH.
A)High pH decreases the activity of all enzymes.
B)High pH increases the activity of all enzymes.
C)High pH changes the shape of all enzymes.
D)High pH does not affect the enzyme, but does lower the availability of substrate.
E)Some enzymes work well at a high pH, some work well at low pH.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
57
Match the transport mechanism to the correct description.
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
transport of specific, targeted molecules, such as hormones, into a cell
a.diffusion
b.facilitated diffusion
c.osmotic pressure
d.active transport
e.receptor-mediated endocytosis
f.osmosis
transport of specific, targeted molecules, such as hormones, into a cell

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
58
The second law of thermodynamics states that ____.
A)matter can be neither created nor destroyed
B)energy can be neither created nor destroyed
C)as energy disperses, entropy increases
D)entropy decreases with time
E)energy is the capacity to do work
A)matter can be neither created nor destroyed
B)energy can be neither created nor destroyed
C)as energy disperses, entropy increases
D)entropy decreases with time
E)energy is the capacity to do work

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck


