Deck 5: Stoichiometry: Quantitative Information About Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/77
Play
Full screen (f)
Deck 5: Stoichiometry: Quantitative Information About Chemical Reactions
1
2 Al(s)+ 6 HCl(aq)→ 2 AlCl3(aq)+ 3 H2(g) According to the equation above,what mass of aluminum is needed to completely react with 2.98 mol of hydrochloric acid?
A) 241 g
B) 36.3 g
C) 26.8 g
D) 2.98 g
E) 80.4 g
A) 241 g
B) 36.3 g
C) 26.8 g
D) 2.98 g
E) 80.4 g
26.8 g
2
Sulfur dioxide will react with water to form sulfurous acid (see balanced equation below). SO2(g)+ H2O(l)→ H2SO3(l)
What mass of sulfur dioxide is needed to prepare 37.11 g of H2SO3(l)?
A) 28.96 g
B) 47.55 g
C) 0.5793 g
D) 0.4521 g
E) 37.11 g
What mass of sulfur dioxide is needed to prepare 37.11 g of H2SO3(l)?
A) 28.96 g
B) 47.55 g
C) 0.5793 g
D) 0.4521 g
E) 37.11 g
28.96 g
3
If 5.00 g Br2 and 1.10 g NH3 react according to the equation below,what is the maximum mass of ammonium bromide produced? 3 Br2( 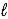 )+ 8 NH3(g)→ 6 NH4Br(s)+ N2(g)
)+ 8 NH3(g)→ 6 NH4Br(s)+ N2(g)
A) 3.06 g
B) 6.13 g
C) 12.9 g
D) 4.74 g
E) 8.43 g
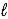 )+ 8 NH3(g)→ 6 NH4Br(s)+ N2(g)
)+ 8 NH3(g)→ 6 NH4Br(s)+ N2(g)A) 3.06 g
B) 6.13 g
C) 12.9 g
D) 4.74 g
E) 8.43 g
4.74 g
4
One step in the isolation of pure rhodium metal (Rh)is the precipitation of rhodium(III)hydroxide from a solution containing rhodium(III)sulfate according to the following balanced chemical equation: Rh2(SO4)3(aq)+ 6NaOH(aq)→ 2Rh(OH)3(s)+ 3Na2SO4(aq)
If 3.10 g of rhodium(III)sulfate reacts with excess sodium hydroxide,what mass of rhodium(III)hydroxide may be produced?
A) 1.93 g
B) 6.20 g
C) 3.10 g
D) 0.483 g
E) 3.86 g
If 3.10 g of rhodium(III)sulfate reacts with excess sodium hydroxide,what mass of rhodium(III)hydroxide may be produced?
A) 1.93 g
B) 6.20 g
C) 3.10 g
D) 0.483 g
E) 3.86 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
5
When strongly heated,boric acid breaks down to boric oxide and water.What mass of boric oxide is formed from the decomposition of 15.0 g of boric acid,B(OH)3? 2 B(OH)3(s)→ B2O3(s)+ 3 H2O(g)
A) 7.50 g
B) 15.0 g
C) 8.44 g
D) 16.9 g
E) 33.8 g
A) 7.50 g
B) 15.0 g
C) 8.44 g
D) 16.9 g
E) 33.8 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
6
Nitric oxide is made from the oxidation of ammonia.What mass of nitric oxide can be made from the reaction of 8.00 g NH3 with 17.0 g O2? 4 NH3(g)+ 5 O2(g)→ 4 NO(g)+ 6 H2O(g)
A) 4.54 g
B) 12.8 g
C) 14.1 g
D) 15.9 g
E) 25.0 g
A) 4.54 g
B) 12.8 g
C) 14.1 g
D) 15.9 g
E) 25.0 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
7
The complete combustion of 0.31 moles of propane,C3H8,will
A) consume 0.31 mol O2 and produce 0.31 mol CO2.
B) consume 0.31 mol O2 and produce 0.62 mol CO2.
C) consume 1.6 mol O2 and produce 0.93 mol CO2.
D) consume 1.6 mol O2 and produce 4.7 mol CO2.
E) consume 1.6 mol O2 and produce 1.2 mol CO2.
A) consume 0.31 mol O2 and produce 0.31 mol CO2.
B) consume 0.31 mol O2 and produce 0.62 mol CO2.
C) consume 1.6 mol O2 and produce 0.93 mol CO2.
D) consume 1.6 mol O2 and produce 4.7 mol CO2.
E) consume 1.6 mol O2 and produce 1.2 mol CO2.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
8
Calculate the number of moles of O2 required to react with phosphorus to produce 5.20 g of P4O6.(Molar mass P4O6 = 219.9 g/mol)
A) 0.0236 mol
B) 0.163 mol
C) 0.0709 mol
D) 0.0473 mol
E) 0.142 mol
A) 0.0236 mol
B) 0.163 mol
C) 0.0709 mol
D) 0.0473 mol
E) 0.142 mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
9
If the complete combustion of an unknown mass of ethylene produces 16.0 g CO2,what mass of ethylene is combusted? C2H4(g)+ 3 O2(g)→ 2 CO2(g)+ 2 H2O(g)
A) 0.182 g
B) 0.364 g
C) 5.10 g
D) 8.00 g
E) 12.6 g
A) 0.182 g
B) 0.364 g
C) 5.10 g
D) 8.00 g
E) 12.6 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
10
Pure copper may be produced by the reaction of copper(I)sulfide with oxygen gas as follows: Cu2S(s)+ O2(g)→ 2Cu(s)+ SO2(g)
What mass of copper(I)sulfide is required in order to prepare 0.750 kg of copper metal?
A) 0.750 kg
B) 0.375 kg
C) 0.564 kg
D) 1.88 kg
E) 0.939 kg
What mass of copper(I)sulfide is required in order to prepare 0.750 kg of copper metal?
A) 0.750 kg
B) 0.375 kg
C) 0.564 kg
D) 1.88 kg
E) 0.939 kg

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
11
How many moles of sodium bromide can be produced from the reaction of 1.03 moles of sodium with 0.650 moles of bromine gas? 2 Na(s)+ Br2(g)→ 2 NaBr(s)
A) 0.650 mol
B) 1.03 mol
C) 1.30 mol
D) 2.06 mol
E) 0.515 mol
A) 0.650 mol
B) 1.03 mol
C) 1.30 mol
D) 2.06 mol
E) 0.515 mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
12
Magnesium reacts with iodine gas at high temperatures to form magnesium iodide.What mass of magnesium iodide,MgI2,can be produced from the reaction of 5.15 g Mg and 50.0 g I2?
A) 29.5 g
B) 44.9 g
C) 54.8 g
D) 55.2 g
E) 58.9 g
A) 29.5 g
B) 44.9 g
C) 54.8 g
D) 55.2 g
E) 58.9 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
13
What mass of iron can be produced from the reaction of 175 kg Fe2O3 with 385 kg CO? Fe2O3(s)+ 3 CO(g)→ 2 Fe(s)+ 3 CO2(g)
A) 2.19 kg
B) 30.6 kg
C) 61.2 kg
D) 122 kg
E) 512 kg
A) 2.19 kg
B) 30.6 kg
C) 61.2 kg
D) 122 kg
E) 512 kg

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
14
How many grams of dioxygen are required to completely burn 4.7 g of C2H5OH?
A) 11 g
B) 16 g
C) 3.3 g
D) 45 g
E) 57 g
A) 11 g
B) 16 g
C) 3.3 g
D) 45 g
E) 57 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
15
The compound P4S3 is used in matches.It reacts with oxygen to produce P4O10 and SO2.The unbalanced chemical equation is shown below. P4S3(s)+ O2(g)→ P4O10(s)+ SO2(g)
What mass of SO2 is produced from the combustion of 0.401 g P4S3?
A) 0.134 g
B) 1.20 g
C) 0.0389 g
D) 0.117 g
E) 0.350 g
What mass of SO2 is produced from the combustion of 0.401 g P4S3?
A) 0.134 g
B) 1.20 g
C) 0.0389 g
D) 0.117 g
E) 0.350 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
16
How many moles of Mg3P2(s)can be produced from the reaction of 0.14 mol Mg(s)with 0.020 mol P4(s)? 6 Mg(s)+ P4(s)→ 2 Mg3P2(s)
A) 0.047 mol
B) 0.14 mol
C) 0.42 mol
D) 0.020 mol
E) 0.040 mol
A) 0.047 mol
B) 0.14 mol
C) 0.42 mol
D) 0.020 mol
E) 0.040 mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
17
If 56.0 g of O2 is mixed with 56.0 g of H2 and the mixture is ignited,what is the maximum mass of water that may be produced?
A) 504 g
B) 63.0 g
C) 56.0 g
D) 112 g
E) 99.6 g
A) 504 g
B) 63.0 g
C) 56.0 g
D) 112 g
E) 99.6 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
18
Potassium hydrogen carbonate decomposes according to the reaction below: 2 KHCO3(s)→ K2CO3(s)+ CO2(g)+ H2O(l)
How many moles of potassium carbonate are produced if 421 g of potassium hydrogen carbonate is heated?
A) 2.10 mol
B) 3.05 mol
C) 17.8 mol
D) 211 mol
E) 4.21 mol
How many moles of potassium carbonate are produced if 421 g of potassium hydrogen carbonate is heated?
A) 2.10 mol
B) 3.05 mol
C) 17.8 mol
D) 211 mol
E) 4.21 mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
19
Hydrogen peroxide decomposes into oxygen and water.What mass of oxygen is formed from the decomposition of 125 g of hydrogen peroxide (H2O2)?
A) 58.8 g
B) 66.4 g
C) 107 g
D) 118 g
E) 125 g
A) 58.8 g
B) 66.4 g
C) 107 g
D) 118 g
E) 125 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
20
Aspirin (C9H8O4)is produced by the reaction of salicylic acid (M = 138.1 g/mol)and acetic anhydride (M = 102.1 g/mol). C7H6O3(s)+ C4H6O3( 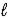 )→ C9H8O4(s)+ C2H4O2(
)→ C9H8O4(s)+ C2H4O2( 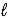 )
)
If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol)can theoretically be obtained?
A) 0.40 g
B) 2.61 g
C) 2.82 g
D) 1.53 g
E) 3.60 g
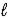 )→ C9H8O4(s)+ C2H4O2(
)→ C9H8O4(s)+ C2H4O2( 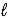 )
)If you react 2.00 g C7H6O3 with 1.60 g C4H6O3,what mass of aspirin (M = 180.2 g/mol)can theoretically be obtained?
A) 0.40 g
B) 2.61 g
C) 2.82 g
D) 1.53 g
E) 3.60 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
21
Nitric oxide,NO,is made from the oxidation of NH3 as follows: 4NH3 + 5O2 → 4NO + 6H2O If 9.0-g of NH3 gives 12.0 g of NO,what is the percent yield of NO?
A) 87 %
B) 47 %
C) 13 %
D) 76 %
E) 25 %
A) 87 %
B) 47 %
C) 13 %
D) 76 %
E) 25 %

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
22
Consider the fermentation reaction of glucose: C6H12O6 → 2C2H5OH + 2CO2
A 1)00-mol sample of C6H12O6 was placed in a vat with 100 g of yeast.If 38.5 g of C2H5OH was obtained,what was the percent yield of C2H5OH?
A) 41.8 %
B) 20.9 %
C) 38.5 %
D) 100 %
E) none of these
A 1)00-mol sample of C6H12O6 was placed in a vat with 100 g of yeast.If 38.5 g of C2H5OH was obtained,what was the percent yield of C2H5OH?
A) 41.8 %
B) 20.9 %
C) 38.5 %
D) 100 %
E) none of these

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
23
A compound consists of only C and F.It contains 24% C by mass.What is the empirical formula of the compound?
A) CF2
B) CF3
C) CF4
D) C2F
E) CF
A) CF2
B) CF3
C) CF4
D) C2F
E) CF

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
24
A 1.295 g sample of a hydrocarbon is burned in an excess of dioxygen,producing 3.553 g CO2 and water.What mass of hydrogen is contained in the original sample?
A) 0.3255 g
B) 2.258 g
C) 4.848 g
D) 1.288 g
E) 0.6475 g
A) 0.3255 g
B) 2.258 g
C) 4.848 g
D) 1.288 g
E) 0.6475 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
25
Of the following,the only empirical formula is
A) C6H12.
B) C4H8.
C) C3H14.
D) H2O2.
E) O3.
A) C6H12.
B) C4H8.
C) C3H14.
D) H2O2.
E) O3.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
26
The reaction of 11.9 g of CHCl3 with excess chlorine produced 10.3 g of CCl4,carbon tetrachloride: 2CHCl3 + 2Cl2 → 2CCl4 + 2HCl
What is the percent yield?
A) 86.6 %
B) 100 %
C) 67.2 %
D) 33.6 %
E) 44.8 %
What is the percent yield?
A) 86.6 %
B) 100 %
C) 67.2 %
D) 33.6 %
E) 44.8 %

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
27
Pure copper may be produced by the reaction of copper(I)sulfide with oxygen gas as follows: Cu2S(s)+ O2(g)→ 2Cu(s)+ SO2(g)
If the reaction of 0.760 kg of copper(I)sulfide with excess oxygen produces 0.310 kg of copper metal,what is the percent yield?
A) 102 %
B) 39.9 %
C) 40.8 %
D) 204 %
E) 51.1 %
If the reaction of 0.760 kg of copper(I)sulfide with excess oxygen produces 0.310 kg of copper metal,what is the percent yield?
A) 102 %
B) 39.9 %
C) 40.8 %
D) 204 %
E) 51.1 %

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
28
The empirical formula of styrene is CH.An experimental determination of the molar mass of styrene yields the value of 104 g/mol.What is the molecular formula of styrene?
A) C5H10
B) C2
C) C8H8
D) C3H8
E) C6H9
A) C5H10
B) C2
C) C8H8
D) C3H8
E) C6H9

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
29
Sulfur trioxide (SO3)is produced from the oxidation of SO2 as follows: 2 SO2 + O2 → 2 SO3 A 23-g sample of SO2 gives 18 g of SO3.Calculate the percent yield of sulfur trioxide,SO3.
A) 10 %
B) 63 %
C) 15 %
D) 29 %
E) 100 %
A) 10 %
B) 63 %
C) 15 %
D) 29 %
E) 100 %

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
30
A 2.288 g sample of a hydrocarbon is burned in an excess of dioxygen,producing 6.277 g CO2 and 5.139 g H2O.What is the empirical formula of the hydrocarbon?
A) CH4
B) CH2
C) C2H3
D) CH3
E) CH
A) CH4
B) CH2
C) C2H3
D) CH3
E) CH

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
31
If 15.0 g of nitrogen (N2)and 2.00 g of hydrogen (H2)react to produce 1.38 g of ammonia (NH3),according to the reaction below,what is the percent yield of the reaction? N2(g)+ 3 H2(g)→ 2 NH3(g)
A) 7.57%
B) 12.2%
C) 8.12%
D) 15.1%
E) 8.17%
A) 7.57%
B) 12.2%
C) 8.12%
D) 15.1%
E) 8.17%

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
32
A 5.95-g sample of AgNO3 is reacted with excess BaCl2 according to the equation 2AgNO3(aq)+ BaCl2(aq)→ 2AgCl(s)+ Ba(NO3)2(aq)
To give 3.57 g of AgCl.What is the percent yield of AgCl?
A) 47.4 %
B) 35.6 %
C) 71.1 %
D) 60.0 %
E) 100 %
To give 3.57 g of AgCl.What is the percent yield of AgCl?
A) 47.4 %
B) 35.6 %
C) 71.1 %
D) 60.0 %
E) 100 %

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
33
Sulfur hexafluoride is produced by reacting elemental sulfur with fluorine gas. S8(s)+ 24 F2(g)→ 8 SF6(g)
What is the percent yield if 18.3 g SF6 is isolated from the reaction of 10.0 g S8 and 30.0 g F2?
A) 40.2%
B) 45.8%
C) 47.6%
D) 54.6%
E) 61.0%
What is the percent yield if 18.3 g SF6 is isolated from the reaction of 10.0 g S8 and 30.0 g F2?
A) 40.2%
B) 45.8%
C) 47.6%
D) 54.6%
E) 61.0%

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
34
The reaction of 0.779 g K with O2 forms 1.417 g potassium superoxide,a substance used in self-contained breathing devices.Determine the formula for potassium superoxide.
A) KO2
B) KO
C) K2O3
D) K2O
E) KO4
A) KO2
B) KO
C) K2O3
D) K2O
E) KO4

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
35
Chlorine was passed over 1.06 g of heated titanium,and 3.40 g of a chloride-containing compound of titanium was obtained.What is the empirical formula of the obtained compound?
A) TiCl2
B) TiCl4
C) TiCl
D) TiCl3
E) Ti2Cl3
A) TiCl2
B) TiCl4
C) TiCl
D) TiCl3
E) Ti2Cl3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
36
A 1.850 g mixture of SrCO3 and SrO is heated.The SrCO3 decomposes to SrO and CO2.What was the mass percentage of SrCO3 in the mixture if the mass after heating is 1.445 g?
A) 26.6%
B) 21.9%
C) 13.7%
D) 73.4%
E) 78.1%
A) 26.6%
B) 21.9%
C) 13.7%
D) 73.4%
E) 78.1%

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
37
Under certain conditions the reaction of ammonia with excess oxygen will produce a 29.5% yield of NO.What mass of NH3 must react with excess oxygen to yield 157 g NO? 4 NH3(g)+ 5 O2(g)→ 4 NO(g)+ 6 H2O(g)
A) 89.1 g
B) 302 g
C) 263 g
D) 26.3 g
E) 938 g
A) 89.1 g
B) 302 g
C) 263 g
D) 26.3 g
E) 938 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
38
Aspirin is produced by the reaction of salicylic acid (M = 138.1 g/mol)and acetic anhydride (M = 102.1 g/mol). C7H6O3(s)+ C4H6O3( 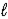 )→ C9H8O4(s)+ C2H4O2(
)→ C9H8O4(s)+ C2H4O2( 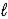 )
)
If 2.04 g of C9H8O4 (M = 180.2 g/mol)is produced from the reaction of 3.03 g C7H6O3 and 4.01 g C4H6O3,what is the percent yield?
A) 28.8%
B) 29.0%
C) 50.9%
D) 51.6%
E) 67.3%
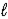 )→ C9H8O4(s)+ C2H4O2(
)→ C9H8O4(s)+ C2H4O2( 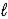 )
)If 2.04 g of C9H8O4 (M = 180.2 g/mol)is produced from the reaction of 3.03 g C7H6O3 and 4.01 g C4H6O3,what is the percent yield?
A) 28.8%
B) 29.0%
C) 50.9%
D) 51.6%
E) 67.3%

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
39
A certain compound has a molar mass of 210 g/mol.Which of the following is a possible empirical formula for this compound?
A) CH2O
B) CHO
C) C2H2O2
D) C2HO
E) C2H2O
A) CH2O
B) CHO
C) C2H2O2
D) C2HO
E) C2H2O

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
40
Complete combustion of a 0.70-mol sample of a hydrocarbon,CxHy,gives 2.80 mol of CO2 and 3.50 mol of H2O.The molecular formula of the original hydrocarbon is
A) C2H8.
B) C4H10.
C) C8H14.
D) C5H5.
E) C4H6.
A) C2H8.
B) C4H10.
C) C8H14.
D) C5H5.
E) C4H6.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
41
What is the molarity of an NaI solution that contains 4.5 g of NaI in 21.0 mL of solution?
A) 1.4 M
B) 0.030 M
C) 0.0047 M
D) 0.00014 M
E) 0.21 M
A) 1.4 M
B) 0.030 M
C) 0.0047 M
D) 0.00014 M
E) 0.21 M

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
42
What is the pH of 4.1 × 10−3 M HCl(aq)?
A) -1.01
B) 2.39
C) 3.50
D) 1.01
E) 5.65
A) -1.01
B) 2.39
C) 3.50
D) 1.01
E) 5.65

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
43
A 1.078 g sample of a compound containing only carbon,hydrogen,and oxygen is burned in an excess of dioxygen,producing 2.451 g CO2 and 1.003 g H2O.What mass of oxygen is contained in the original sample?
A) 0.2970 g
B) 0.3531 g
C) 1.373 g
D) 1.447 g
E) 0.07480 g
A) 0.2970 g
B) 0.3531 g
C) 1.373 g
D) 1.447 g
E) 0.07480 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
44
A mass of 0.4113 g of an unknown acid,HA,is titrated with sodium hydroxide,NaOH.If the acid reacts with 28.10 mL of 0.1055 M aqueous sodium hydroxide,what is the molar mass of the acid?
A) 2.965 × 10-3 g/mol
B) 9.128 g/mol
C) 138.7 g/mol
D) 337.3 g/mol
E) 820.7 g/mol
A) 2.965 × 10-3 g/mol
B) 9.128 g/mol
C) 138.7 g/mol
D) 337.3 g/mol
E) 820.7 g/mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
45
The combustion of 0.0272 mole of a hydrocarbon produces 1.9609 g H2O and 3.5927 g CO2.What is the molar mass of the hydrocarbon?
A) 16.0 g/mol
B) 30.1 g/mol
C) 44.1 g/mol
D) 72.2 g/mol
E) 92.1 g/mol
A) 16.0 g/mol
B) 30.1 g/mol
C) 44.1 g/mol
D) 72.2 g/mol
E) 92.1 g/mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
46
A dilute solution is prepared by transferring 35.00 mL of a 0.6363 M stock solution to a 900.0 mL volumetric flask and diluting to mark.What is the molarity of this dilute solution?
A) 0.02474 M
B) 0.04949 M
C) 0.1636 M
D) 0.006186 M
E) 0.3182 M
A) 0.02474 M
B) 0.04949 M
C) 0.1636 M
D) 0.006186 M
E) 0.3182 M

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
47
Zn reacts with hydrochloric acid. Zn(s)+ 2 HCl(aq)→ ZnCl2(aq)+ H2(g)
What volume of 3.05 M HCl(aq)will react with 25.0 g Zn(s)?
A) 0.251 L
B) 4.01 L
C) 0.125 L
D) 0.0627 L
E) 2.33 L
What volume of 3.05 M HCl(aq)will react with 25.0 g Zn(s)?
A) 0.251 L
B) 4.01 L
C) 0.125 L
D) 0.0627 L
E) 2.33 L

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
48
In order to dilute 77.1 mL of 0.778 M HCl to 0.100 M,the volume of water that must be added is
A) 67.2 mL.
B) 9.91 mL.
C) 6 × 102 mL.
D) 1.01 × 10-3 mL.
E) 5.23 × 102 mL.
A) 67.2 mL.
B) 9.91 mL.
C) 6 × 102 mL.
D) 1.01 × 10-3 mL.
E) 5.23 × 102 mL.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
49
How many moles of sulfate ions are there in a 0.650-L solution of 0.312 M Al2(SO4)3?
A) 0.203 mol
B) 0.608 mol
C) 6.25 mol
D) 0.0676 mol
E) 1.44 mol
A) 0.203 mol
B) 0.608 mol
C) 6.25 mol
D) 0.0676 mol
E) 1.44 mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
50
The pH of an aqueous sodium hydroxide solution is 12.83.What is the hydronium ion concentration of this solution?
A) 1.5 × 10-13 M
B) 6.7 × 10-2 M
C) 1.2 M
D) 6.7 × 1012 M
E) 2.7 × 10-6 M
A) 1.5 × 10-13 M
B) 6.7 × 10-2 M
C) 1.2 M
D) 6.7 × 1012 M
E) 2.7 × 10-6 M

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
51
A 2.197 g sample of a compound containing only carbon,hydrogen,and oxygen is burned in an excess of dioxygen,producing 4.197 g CO2 and 2.578 g H2O.What is the empirical formula of the compound?
A) CHO
B) C2H6O
C) C3H6O
D) C3H4O
E) C3H8O3
A) CHO
B) C2H6O
C) C3H6O
D) C3H4O
E) C3H8O3

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
52
Naphthalene,a hydrocarbon,has an approximate molar mass of 128 g/mole.If the combustion of 0.6400 g of naphthalene produces 0.3599 g of H2O and 2.1977 g of CO2,what is the molecular formula of this compound?
A) C8H32
B) C9H18
C) C9H20
D) C10H8
E) C11H7
A) C8H32
B) C9H18
C) C9H20
D) C10H8
E) C11H7

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
53
What volume of 1.27 M HCl is required to prepare 197.4 mL of 0.456 M HCl?
A) 5.5 × 102 mL
B) 3.41 × 102 mL
C) 70.9 mL
D) 0.0141 mL
E) 1.14 × 102 mL
A) 5.5 × 102 mL
B) 3.41 × 102 mL
C) 70.9 mL
D) 0.0141 mL
E) 1.14 × 102 mL

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
54
Soft drink bottles are made of polyethylene terephthalate (PET),a polymer composed of carbon,hydrogen,and oxygen.If 1.9022 g PET is burned in oxygen it produces 0.6585 g H2O and 4.0216 g CO2.What is the empirical formula of PET?
A) CHO
B) CH7O5
C) C5H7O
D) C8H10O
E) C10H8O5
A) CHO
B) CH7O5
C) C5H7O
D) C8H10O
E) C10H8O5

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
55
The pH of a vinegar solution is 4.15.What is the H3O+ concentration of the solution?
A) 7.1 × 10-5 M
B) 1.6 × 10-2 M
C) 0.62 M
D) 1.4 M
E) 1.4 × 104 M
A) 7.1 × 10-5 M
B) 1.6 × 10-2 M
C) 0.62 M
D) 1.4 M
E) 1.4 × 104 M

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
56
An aqueous nitric acid solution has a pH of 2.15.What mass of nitric acid,HNO3,is present in 20.0 L of this solution?
A) 0.11 g
B) 0.022 g
C) 3.7 g
D) 6.8 g
E) 8.9 g
A) 0.11 g
B) 0.022 g
C) 3.7 g
D) 6.8 g
E) 8.9 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
57
What mass of Na2CO3 is present in 0.700 L of a 0.396 M Na2CO3 solution?
A) 29.4 g
B) 74.2 g
C) 42.0 g
D) 187 g
E) 60.0 g
A) 29.4 g
B) 74.2 g
C) 42.0 g
D) 187 g
E) 60.0 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
58
What minimum mass of cobalt (II)nitrate must be added to 60.0 mL of a 0.0999 M phosphate solution in order to completely precipitate all of the phosphate as solid cobalt (II)phosphate? 2PO43-(aq)+ 3Co(NO3)2(aq)→ Co3(PO4)2(s)+ 6NO3-(aq)
A) 1.10 g
B) 3.29 g
C) 0.731 g
D) 0.366 g
E) 1.64 g
A) 1.10 g
B) 3.29 g
C) 0.731 g
D) 0.366 g
E) 1.64 g

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
59
Polyethylene is a polymer consisting of only carbon and hydrogen.If 2.300 g of the polymer is burned in oxygen it produces 2.955 g of H2O and 7.217 g of CO2.Determine the empirical formula of polyethylene.
A) CH
B) CH2
C) C2H3
D) C5H8
E) C7H8
A) CH
B) CH2
C) C2H3
D) C5H8
E) C7H8

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
60
What volume of 0.742 M Na2CO3 solution contains 44.9 g of Na2CO3?
A) 0.314 L
B) 6.41 × 103 L
C) 0.571 L
D) 3.53 × 103 L
E) 1.75 L
A) 0.314 L
B) 6.41 × 103 L
C) 0.571 L
D) 3.53 × 103 L
E) 1.75 L

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is true about the actual yield of a product?
A) It is almost always less than the theoretical yield.
B) It is always equal to the theoretical yield of the reaction.
C) It is always greater than the theoretical yield.
D) It is obtained from a stoichiometry calculation.
E) It is equal to the molar mass of the product.
A) It is almost always less than the theoretical yield.
B) It is always equal to the theoretical yield of the reaction.
C) It is always greater than the theoretical yield.
D) It is obtained from a stoichiometry calculation.
E) It is equal to the molar mass of the product.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
62
The reaction of 5.07 g N2 with 0.722 g H2 produces 1.27 g NH3.The percent yield of this reaction is ________.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
63
Phosphorus trichloride,PCl3,can be produced from the reaction below:
P4(s)+ 6 Cl2(g)→ 4 PCl3(g)
If 1.00 g of phosphorus,P4,reacts with 1.00 g of chlorine,Cl2,which reactant is the limiting reactant and what mass of product may be produced?
P4(s)+ 6 Cl2(g)→ 4 PCl3(g)
If 1.00 g of phosphorus,P4,reacts with 1.00 g of chlorine,Cl2,which reactant is the limiting reactant and what mass of product may be produced?

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
64
In combustion analysis,the gases produced by the combustion of a hydrocarbon are passed through a tube containing finely divided sodium hydroxide supported on asbestos.The purpose of the sodium hydroxide is to absorb ________ produced by the combustion reaction.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
65
The following equation: A = ε × 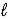 × C (where
× C (where 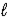 is path length and C is concentration)is known as the _____-Lambert law.
is path length and C is concentration)is known as the _____-Lambert law.
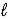 × C (where
× C (where 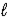 is path length and C is concentration)is known as the _____-Lambert law.
is path length and C is concentration)is known as the _____-Lambert law.
Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
66
The percent yield of a chemical reaction is calculated by dividing the actual yield by the ________ yield and multiplying by 100%.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
67
The pH of purified water (or of a neutral solution)is _____.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
68
Calibration of a spectrophotometer using a series of green dye containing solutions provides a set of data which follows the Beer-Lambert Law,with a trendline of y = 1.398 × 103x (y-axis = absorbance,x-axis = molar concentration).What is the molar concentration of a solution with an absorbance of 0.8427?
A) 0.6028 M
B) 0.8427 M
C) 1.187 M
D) 0.7153 M
E) 0.1573
A) 0.6028 M
B) 0.8427 M
C) 1.187 M
D) 0.7153 M
E) 0.1573

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
69
A solution of sodium oxalate (Na2C2O4)in acidic solution is titrated with a solution of potassium permanganate (KMnO4)according to the following balanced chemical equation: 2KMnO4(aq)+ 8H2SO4(aq)+ 5Na2C2O4(aq)→ 2MnSO4(aq)+ 8H2O(l)+ 10CO2(g)+ 5Na2SO4(aq)+ K2SO4(aq)
What volume of 0.0206 M KMnO4 is required to titrate 0.176 g of Na2C2O4 dissolved in 50.0 mL of solution?
A) 3.42 mL
B) 8.54 mL
C) 25.5 mL
D) 63.8 mL
E) 50.0 mL
What volume of 0.0206 M KMnO4 is required to titrate 0.176 g of Na2C2O4 dissolved in 50.0 mL of solution?
A) 3.42 mL
B) 8.54 mL
C) 25.5 mL
D) 63.8 mL
E) 50.0 mL

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
70
The reaction of HCl with NaOH is represented by the equation HCl(aq)+ NaOH(aq)→ NaCl(aq)+ H2O(l)
What volume of 0.614 M HCl is required to titrate 18.8 mL of 0.619 M NaOH?
A) 7.15 mL
B) 1.63 mL
C) 19.0 mL
D) 18.8 mL
E) 18.6 mL
What volume of 0.614 M HCl is required to titrate 18.8 mL of 0.619 M NaOH?
A) 7.15 mL
B) 1.63 mL
C) 19.0 mL
D) 18.8 mL
E) 18.6 mL

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
71
The numbers preceding the formulas in chemical equations are referred to as the _____ coefficients.

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
72
An unknown diprotic acid (H2A)requires 31.81 mL of 0.109 M NaOH to completely neutralize a 0.685 g sample.Calculate the approximate molar mass of the acid.
A) 577 g/mol
B) 395 g/mol
C) 198 g/mol
D) 99 g/mol
E) 288 g/mol
A) 577 g/mol
B) 395 g/mol
C) 198 g/mol
D) 99 g/mol
E) 288 g/mol

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
73
Molarity is defined as the _____.
A) number of mole equivalents per liter of solution
B) amount of solute per liter of solution
C) mass of a constituent divided by the volume of the mixture
D) ratio of one substance with the mass of the total mixture
E) amount of a constituent divided by the total amount of all constituents in a mixture
A) number of mole equivalents per liter of solution
B) amount of solute per liter of solution
C) mass of a constituent divided by the volume of the mixture
D) ratio of one substance with the mass of the total mixture
E) amount of a constituent divided by the total amount of all constituents in a mixture

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
74
If 0.1800 g of impure soda ash (Na2CO3)is titrated with 15.66 mL of 0.1082 M HCl,what is the percent purity of the soda ash? Na2CO3(aq)+ 2 HCl(aq)→ 2 NaCl(aq)+ H2O( 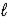 )+ CO2(g)
)+ CO2(g)
A) 17.96%
B) 49.89%
C) 50.11%
D) 94.13%
E) 99.77%
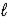 )+ CO2(g)
)+ CO2(g)A) 17.96%
B) 49.89%
C) 50.11%
D) 94.13%
E) 99.77%

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
75
A 30.00-mL sample of a weak monoprotic acid is titrated with 0.0915 M NaOH.At the endpoint,it is found that 45.98 mL of titrant was used.What was the concentration of the weak acid?
A) 0.140 M
B) 4.21 M
C) 0.0915 M
D) 6.63 × 10-5 M
E) 0.0597 M
A) 0.140 M
B) 4.21 M
C) 0.0915 M
D) 6.63 × 10-5 M
E) 0.0597 M

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
76
An impure sample of benzoic acid (C6H5COOH,122.12 g/mol)is titrated with 0.5360 M NaOH.A 1.888-g sample requires 20.50 mL of titrant to reach the endpoint.What is the percent by mass of benzoic acid in the sample? C6H5COOH(aq)+ NaOH(aq)→ NaC6H5COO(aq)+ H2O(l)
A) 0.008998 %
B) 1.099 %
C) 100.0 %
D) 8.998 %
E) 71.06 %
A) 0.008998 %
B) 1.099 %
C) 100.0 %
D) 8.998 %
E) 71.06 %

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following is the correct representation for absorbance of a sample?
A) A = -log T
B) A = log (-T)
C) A = 10T
D) A = 10-T
E) A = 10-1/T
A) A = -log T
B) A = log (-T)
C) A = 10T
D) A = 10-T
E) A = 10-1/T

Unlock Deck
Unlock for access to all 77 flashcards in this deck.
Unlock Deck
k this deck


