Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
An unknown diprotic acid (H2A)requires 31.81 mL of 0.109 M NaOH to completely neutralize a 0.685 g sample.Calculate the approximate molar mass of the acid.
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
B
The compound P4S3 is used in matches.It reacts with oxygen to produce P4O10 and SO2.The unbalanced chemical equation is shown below. P4S3(s)+ O2(g)→ P4O10(s)+ SO2(g)
What mass of SO2 is produced from the combustion of 0.401 g P4S3?
Free
(Multiple Choice)
4.7/5  (40)
(40)
Correct Answer:
E
A solution of sodium oxalate (Na2C2O4)in acidic solution is titrated with a solution of potassium permanganate (KMnO4)according to the following balanced chemical equation: 2KMnO4(aq)+ 8H2SO4(aq)+ 5Na2C2O4(aq)→ 2MnSO4(aq)+ 8H2O(l)+ 10CO2(g)+ 5Na2SO4(aq)+ K2SO4(aq)
What volume of 0.0206 M KMnO4 is required to titrate 0.176 g of Na2C2O4 dissolved in 50.0 mL of solution?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
C
Hydrogen peroxide decomposes into oxygen and water.What mass of oxygen is formed from the decomposition of 125 g of hydrogen peroxide (H2O2)?
(Multiple Choice)
4.9/5  (37)
(37)
Calibration of a spectrophotometer using a series of green dye containing solutions provides a set of data which follows the Beer-Lambert Law,with a trendline of y = 1.398 × 103x (y-axis = absorbance,x-axis = molar concentration).What is the molar concentration of a solution with an absorbance of 0.8427?
(Multiple Choice)
4.8/5  (38)
(38)
One step in the isolation of pure rhodium metal (Rh)is the precipitation of rhodium(III)hydroxide from a solution containing rhodium(III)sulfate according to the following balanced chemical equation: Rh2(SO4)3(aq)+ 6NaOH(aq)→ 2Rh(OH)3(s)+ 3Na2SO4(aq)
If 3.10 g of rhodium(III)sulfate reacts with excess sodium hydroxide,what mass of rhodium(III)hydroxide may be produced?
(Multiple Choice)
4.9/5  (37)
(37)
How many moles of sodium bromide can be produced from the reaction of 1.03 moles of sodium with 0.650 moles of bromine gas? 2 Na(s)+ Br2(g)→ 2 NaBr(s)
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following is true about the actual yield of a product?
(Multiple Choice)
4.9/5  (38)
(38)
In combustion analysis,the gases produced by the combustion of a hydrocarbon are passed through a tube containing finely divided sodium hydroxide supported on asbestos.The purpose of the sodium hydroxide is to absorb ________ produced by the combustion reaction.
(Short Answer)
4.8/5  (38)
(38)
How many moles of Mg3P2(s)can be produced from the reaction of 0.14 mol Mg(s)with 0.020 mol P4(s)? 6 Mg(s)+ P4(s)→ 2 Mg3P2(s)
(Multiple Choice)
4.9/5  (38)
(38)
Aspirin is produced by the reaction of salicylic acid (M = 138.1 g/mol)and acetic anhydride (M = 102.1 g/mol). C7H6O3(s)+ C4H6O3( 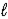 )→ C9H8O4(s)+ C2H4O2(
)→ C9H8O4(s)+ C2H4O2( 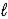 )
If 2.04 g of C9H8O4 (M = 180.2 g/mol)is produced from the reaction of 3.03 g C7H6O3 and 4.01 g C4H6O3,what is the percent yield?
)
If 2.04 g of C9H8O4 (M = 180.2 g/mol)is produced from the reaction of 3.03 g C7H6O3 and 4.01 g C4H6O3,what is the percent yield?
(Multiple Choice)
4.7/5  (42)
(42)
The pH of an aqueous sodium hydroxide solution is 12.83.What is the hydronium ion concentration of this solution?
(Multiple Choice)
4.8/5  (41)
(41)
What is the molarity of an NaI solution that contains 4.5 g of NaI in 21.0 mL of solution?
(Multiple Choice)
4.8/5  (36)
(36)
Sulfur dioxide will react with water to form sulfurous acid (see balanced equation below). SO2(g)+ H2O(l)→ H2SO3(l)
What mass of sulfur dioxide is needed to prepare 37.11 g of H2SO3(l)?
(Multiple Choice)
4.8/5  (33)
(33)
Under certain conditions the reaction of ammonia with excess oxygen will produce a 29.5% yield of NO.What mass of NH3 must react with excess oxygen to yield 157 g NO? 4 NH3(g)+ 5 O2(g)→ 4 NO(g)+ 6 H2O(g)
(Multiple Choice)
4.8/5  (39)
(39)
Calculate the number of moles of O2 required to react with phosphorus to produce 5.20 g of P4O6.(Molar mass P4O6 = 219.9 g/mol)
(Multiple Choice)
4.8/5  (35)
(35)
A compound consists of only C and F.It contains 24% C by mass.What is the empirical formula of the compound?
(Multiple Choice)
4.9/5  (32)
(32)
The percent yield of a chemical reaction is calculated by dividing the actual yield by the ________ yield and multiplying by 100%.
(Short Answer)
4.9/5  (32)
(32)
A 2.197 g sample of a compound containing only carbon,hydrogen,and oxygen is burned in an excess of dioxygen,producing 4.197 g CO2 and 2.578 g H2O.What is the empirical formula of the compound?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 1 - 20 of 77
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)