Deck 3: Atoms, molecules, and Ions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/101
Play
Full screen (f)
Deck 3: Atoms, molecules, and Ions
1
Which of the following atoms contains the largest number of neutrons?
A)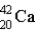
B)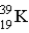
C)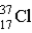
D)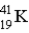
E)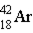
A)
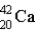
B)
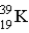
C)
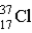
D)
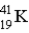
E)
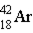
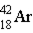
2
Atomic number is equal to the _____ in the nucleus of an atom.
A) number of electrons
B) number of protons
C) number of protons minus the number of neutrons
D) sum of the number of electrons and neutrons
E) sum of the number of neutrons and protons
A) number of electrons
B) number of protons
C) number of protons minus the number of neutrons
D) sum of the number of electrons and neutrons
E) sum of the number of neutrons and protons
number of protons
3
How many neutrons are there in an atom of copper-63 ?
A) 29
B) 2
C) 92
D) 63
E) 34
A) 29
B) 2
C) 92
D) 63
E) 34
34
4
Electrons surround the nucleus and account for the majority of an atom's volume.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
5
How many protons,neutrons,and electrons are present in an atom of iron-59?
A) 26 protons,33 neutrons,and 59 electrons
B) 26 protons,33 neutrons,and 33 electrons
C) 26 protons,33 neutrons,and 26 electrons
D) 59 protons,26 neutrons,and 59 electrons
E) 59 protons,26 neutrons,and 26 electrons
A) 26 protons,33 neutrons,and 59 electrons
B) 26 protons,33 neutrons,and 33 electrons
C) 26 protons,33 neutrons,and 26 electrons
D) 59 protons,26 neutrons,and 59 electrons
E) 59 protons,26 neutrons,and 26 electrons

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following atomic symbols represents an isotope of 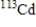 ?
?
A)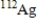
B)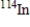
C)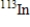
D)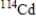
E)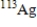
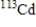 ?
?A)
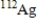
B)
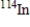
C)
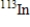
D)
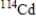
E)
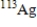

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following atoms contains the least protons?
A) (232Th)
B) (231Pa)
C) (245Pu)
D) (238U)
E) (232Pa)
A) (232Th)
B) (231Pa)
C) (245Pu)
D) (238U)
E) (232Pa)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
8
How many protons are there in an atom of scandium-45?
A) 25
B) 66
C) 20
D) 21
E) 24
A) 25
B) 66
C) 20
D) 21
E) 24

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
9
Two isotopes of a given element will have the same number of _____,but a different number of _____ in their nucleus.
A) protons; electrons
B) electrons; protons
C) protons; neutrons
D) neutrons; protons
E) electrons; neutrons
A) protons; electrons
B) electrons; protons
C) protons; neutrons
D) neutrons; protons
E) electrons; neutrons

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following atoms contains more protons than neutrons?
A)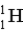
B)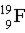
C)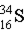
D)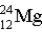
E)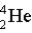
A)
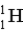
B)
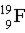
C)
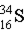
D)
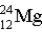
E)
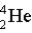

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
11
If two different isotopes have the same atomic number,it means that _____.
A) they have the same atomic mass
B) they have the same atomic number
C) they have the same number of protons
D) they have the same number of electrons
E) they have the same number of neutrons
A) they have the same atomic mass
B) they have the same atomic number
C) they have the same number of protons
D) they have the same number of electrons
E) they have the same number of neutrons

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
12
Arrange the subatomic particles in order of their increasing mass.
A) Electron mass = proton mass = neutron mass
B) Electron mass = neutron mass < proton mass
C) Electron mass = proton mass < neutron mass
D) Electron mass < proton mass < neutron mass
E) Electron mass < proton mass = neutron mass
A) Electron mass = proton mass = neutron mass
B) Electron mass = neutron mass < proton mass
C) Electron mass = proton mass < neutron mass
D) Electron mass < proton mass < neutron mass
E) Electron mass < proton mass = neutron mass

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following elements does 'X' represent in 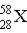 ?
?
A) Ni
B) Zn
C) Rn
D) Ce
E) Pd
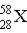 ?
?A) Ni
B) Zn
C) Rn
D) Ce
E) Pd

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
14
What is the mass of chlorine-35 relative to carbon-12?
A) 0.657
B) 0.522
C) 1.52
D) 2.92
E) 23
A) 0.657
B) 0.522
C) 1.52
D) 2.92
E) 23

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
15
The masses of isotopes and their abundances are determined experimentally using _____.
A) a mass spectrometer
B) an analytical balance
C) a centrifuge
D) distillation
E) electrolysis
A) a mass spectrometer
B) an analytical balance
C) a centrifuge
D) distillation
E) electrolysis

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
16
Atoms are made of three subatomic particles.What are these particles and their charges?
A) proton (+1),neutron (0),and electron (−1)
B) proton (−1),neutron (+1),and electron (0)
C) proton (+1),neutron (−1),and electron (0)
D) proton (0),neutron (+1),and electron (−1)
E) proton (−1),neutron (0),and electron (+1)
A) proton (+1),neutron (0),and electron (−1)
B) proton (−1),neutron (+1),and electron (0)
C) proton (+1),neutron (−1),and electron (0)
D) proton (0),neutron (+1),and electron (−1)
E) proton (−1),neutron (0),and electron (+1)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is the correct atomic symbol for an element with 17 protons and 18 neutrons?
A)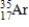
B)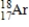
C)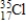
D)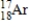
E)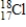
A)
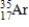
B)
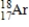
C)
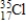
D)
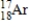
E)
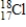

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements is true concerning 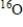 and
and 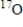 ?
?
A) They have the same number of neutrons.
B) They are isotopes.
C) They have the same relative atomic mass.
D) They have the same mass number.
E) They have different chemical properties.
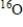 and
and 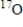 ?
?A) They have the same number of neutrons.
B) They are isotopes.
C) They have the same relative atomic mass.
D) They have the same mass number.
E) They have different chemical properties.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
19
An atom that has the same number of neutrons as nickel-59 is _____.
A)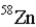
B)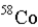
C)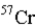
D)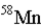
E)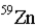
A)
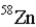
B)
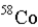
C)
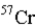
D)
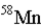
E)
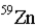

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is the correct atomic symbol for an element that has 30 neutrons and a mass number of 55?
A) At
B) Zn
C) Co
D) Mn
E) Cs
A) At
B) Zn
C) Co
D) Mn
E) Cs

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following elements is present in the third period of group 7A?
A) S
B) Cl2
C) I2
D) H2
E) Ar
A) S
B) Cl2
C) I2
D) H2
E) Ar

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
22
A sample of an element consists of two isotopes.The percent abundance of one of the isotopes is 75.0%.What is the percent abundance of the other isotope?
A) 62.5%
B) 37.5%
C) 12.5%
D) 75.0%
E) 25.0%
A) 62.5%
B) 37.5%
C) 12.5%
D) 75.0%
E) 25.0%

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
23
Copper has an atomic weight of 63.55 u.If 69.17% of copper exists as 63Cu (62.93960 u),what is the identity and the atomic mass of the other isotope?
A) Cu-64; 63.82 u
B) Cu-64; 64.16 u
C) Cu-65; 64.16 u
D) Cu-65; 64.92 u
E) Cu-66; 65.91 u
A) Cu-64; 63.82 u
B) Cu-64; 64.16 u
C) Cu-65; 64.16 u
D) Cu-65; 64.92 u
E) Cu-66; 65.91 u

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following elements is not a metalloid?
A) Boron
B) Selenium
C) Germanium
D) Arsenic
E) Silicon
A) Boron
B) Selenium
C) Germanium
D) Arsenic
E) Silicon

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
25
What is the common name of the group that has an element with 38 protons in its nucleus?
A) Transition metals
B) Halogens
C) Noble gases
D) Alkaline earth metals
E) Alkali metals
A) Transition metals
B) Halogens
C) Noble gases
D) Alkaline earth metals
E) Alkali metals

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
26
Silver has two stable isotopes with masses of 106.90509 u and 108.9047 u.The atomic weight of silver is 107.868 u.What is the percent abundance of each isotope?
A) 50.0% 107Ag and 50.0% 109Ag
B) 51.8% 107Ag and 48.2% 109Ag
C) 55.4% 107Ag and 44.6% 109Ag
D) 48.2% 107Ag and 51.8% 109Ag
E) 44.6% 107Ag and 55.4% 109Ag
A) 50.0% 107Ag and 50.0% 109Ag
B) 51.8% 107Ag and 48.2% 109Ag
C) 55.4% 107Ag and 44.6% 109Ag
D) 48.2% 107Ag and 51.8% 109Ag
E) 44.6% 107Ag and 55.4% 109Ag

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
27
Naturally occurring element X exists in three isotopic forms: X-28 (27.979 u,90.64% abundance),X-29 (28.976 u,4.00% abundance),and X-30 (29.974 u,5.36% abundance).Calculate the atomic weight of X.
A) 29.64 u
B) 28.13 u
C) 28.99 u
D) 29.83 u
E) 27.15 u
A) 29.64 u
B) 28.13 u
C) 28.99 u
D) 29.83 u
E) 27.15 u

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following statements is not true about the element vanadium?
A) It is a metal.
B) It has chemical and physical properties most similar to krypton.
C) It is in period 4.
D) It has chemical and physical properties most similar to silver.
E) It is in group 5B.
A) It is a metal.
B) It has chemical and physical properties most similar to krypton.
C) It is in period 4.
D) It has chemical and physical properties most similar to silver.
E) It is in group 5B.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
29
The mass spectrum of an element with two naturally occurring isotopes is shown below.What is the best estimate of the element's (average)atomic weight? 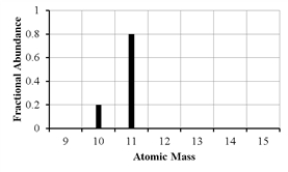
A) 10 amu
B) 11 amu
C) 10.8 amu
D) 10.2 amu
E) 10.5 amu

A) 10 amu
B) 11 amu
C) 10.8 amu
D) 10.2 amu
E) 10.5 amu

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following elements is present in the fourth period of Group 3A?
A) Sb
B) Ga
C) In
D) Si
E) Tl
A) Sb
B) Ga
C) In
D) Si
E) Tl

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following elements is present in fourth period of group 3?
A) Flourine
B) Chlorine
C) Barium
D) Argon
E) Sodium
A) Flourine
B) Chlorine
C) Barium
D) Argon
E) Sodium

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
32
The element chlorine has two stable isotopes,chlorine-35 with an atomic mass of 34.97 u and chlorine-37 with an atomic mass of 36.97 u.From the atomic weight found on the periodic table,one can conclude that _____.
A) both isotopes have the same percent natural abundance
B) there is an isotope of nitrogen with an atomic mass of 35.45 u
C) chlorine-35 has the highest percent natural abundance
D) chlorine-37 has the highest percent natural abundance
E) there is an isotope of nitrogen with an atomic mass of 37.95 u
A) both isotopes have the same percent natural abundance
B) there is an isotope of nitrogen with an atomic mass of 35.45 u
C) chlorine-35 has the highest percent natural abundance
D) chlorine-37 has the highest percent natural abundance
E) there is an isotope of nitrogen with an atomic mass of 37.95 u

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following elements belongs to the alkaline earth metals series?
A) Barium
B) Potassium
C) phosphorus
D) Bromine
E) Argon
A) Barium
B) Potassium
C) phosphorus
D) Bromine
E) Argon

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
34
Lithium has two naturally occurring isotopes,6Li and 7Li.The atomic weight of lithium is 6.941.Which of the following statements concerning the relative abundance of each isotope is correct?
A) The abundance of 7Li is greater than 6Li.
B) The abundance of 7Li is less than 6Li.
C) The abundance of 6Li is equal to the abundance of 7Li.
D) Not enough data is provided to determine the correct answer.
E) Based on the atomic mass,only 7Li occurs naturally.
A) The abundance of 7Li is greater than 6Li.
B) The abundance of 7Li is less than 6Li.
C) The abundance of 6Li is equal to the abundance of 7Li.
D) Not enough data is provided to determine the correct answer.
E) Based on the atomic mass,only 7Li occurs naturally.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following groups of periodic table contains only nonmetals?
A) 2A
B) 3A
C) 5A
D) 6A
E) 7A
A) 2A
B) 3A
C) 5A
D) 6A
E) 7A

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
36
An element consists of three isotopes.The abundance of one isotope is 92.21% and its atomic mass is 27.97693 u.The abundance of the second isotope is 4.70% and its atomic mass is 28.97649 u.The atomic mass of the third isotope is 29.97376 u.What is the atomic weight of the element?
A) 28.09 u
B) 28.98 u
C) 28.96 u
D) 29.87 u
E) 29.07 u
A) 28.09 u
B) 28.98 u
C) 28.96 u
D) 29.87 u
E) 29.07 u

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
37
The formula of acetic acid,CH3CO2H,is an example of a(n)_____.
A) condensed formula
B) empirical formula
C) structural formula
D) ionic compound formula
E) elemental formula
A) condensed formula
B) empirical formula
C) structural formula
D) ionic compound formula
E) elemental formula

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
38
The elements in groups 1A-8A are known as the _____.
A) main group elements
B) transition metals
C) halogens
D) metalloids or semimetals
E) noble gases
A) main group elements
B) transition metals
C) halogens
D) metalloids or semimetals
E) noble gases

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
39
A certain element consists of two stable isotopes.The first has a mass of 14.0031 amu and a percent natural abundance of 99.63%.The second has a mass of 15.001 amu and a percent natural abundance of 0.37%.What is the atomic weight of the element?
A) 13.95 amu
B) 14.00 amu
C) 14.01 amu
D) 14.50 amu
E) 19.50 amu
A) 13.95 amu
B) 14.00 amu
C) 14.01 amu
D) 14.50 amu
E) 19.50 amu

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
40
Rubidium has two naturally occurring isotopes.The atomic weight of Rb is 85.4678 u.If 72.15% of Rb is found as Rb-85 (84.9117 u),what is the mass of the other isotope?
A) 0.56 u
B) 85.68 u
C) 86.68 u
D) 86.02 u
E) 83.47 u
A) 0.56 u
B) 85.68 u
C) 86.68 u
D) 86.02 u
E) 83.47 u

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
41
What is the charge on the sodium ion in Na3P?
A) 3-
B) 1-
C) 0
D) 1+
E) 3+
A) 3-
B) 1-
C) 0
D) 1+
E) 3+

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
42
Scandium(III)sulfite is an ionic compound formed from Sc3+ and SO32-.What is the correct way to represent the formula?
A) ScSO3+
B) Sc(SO3)2-
C) Sc3+SO32-
D) Sc2(SO3)3
E) Sc5(SO3)8
A) ScSO3+
B) Sc(SO3)2-
C) Sc3+SO32-
D) Sc2(SO3)3
E) Sc5(SO3)8

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following atoms of elements is most likely to form a 2+ ion?
A) Scandium
B) Calcium
C) Aluminum
D) Oxygen
E) Fluorine
A) Scandium
B) Calcium
C) Aluminum
D) Oxygen
E) Fluorine

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following formulas is not correct?
A) AlPO4
B) KClO4
C) CaS
D) Na(NO3)2
E) Na2HPO4
A) AlPO4
B) KClO4
C) CaS
D) Na(NO3)2
E) Na2HPO4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is the correct formula for an ionic compound that contains barium ions and carbonate ions?
A) BaCO3
B) Ba(HCO3)2
C) Ba2CO3
D) Ba2C
E) Ba(CO3)2
A) BaCO3
B) Ba(HCO3)2
C) Ba2CO3
D) Ba2C
E) Ba(CO3)2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
46
What charge is likely possible on a monatomic silver cation?
A) 2-
B) 1-
C) 1+
D) 2+
E) 3+
A) 2-
B) 1-
C) 1+
D) 2+
E) 3+

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is the correct formula for manganese(III)sulfate?
A) MnSO4
B) Mn2SO4
C) Mn3(SO4)2
D) Mn2(SO4)3
E) Mn(SO4)2
A) MnSO4
B) Mn2SO4
C) Mn3(SO4)2
D) Mn2(SO4)3
E) Mn(SO4)2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following sets of ions is present in sodium sulfate,Na2SO4?
A) Na+,S2−,and O2−
B) Na+,S2+,and O2−
C) Na+ and SO42−
D) Na+,S2−,and O2+
E) Na+ and SO4−
A) Na+,S2−,and O2−
B) Na+,S2+,and O2−
C) Na+ and SO42−
D) Na+,S2−,and O2+
E) Na+ and SO4−

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is the correct name for the compound NH4NO3?
A) Ammonia hydrogen nitrate
B) Ammonia hydrogen nitride
C) Ammonium nitric acid
D) Ammonium nitrate
E) Ammonium nitride
A) Ammonia hydrogen nitrate
B) Ammonia hydrogen nitride
C) Ammonium nitric acid
D) Ammonium nitrate
E) Ammonium nitride

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is the correct formula for potassium dihydrogen phosphate?
A) KH2PO4
B) K2HPO4
C) K2H2PO4
D) K3H2PO4
E) KH2P
A) KH2PO4
B) K2HPO4
C) K2H2PO4
D) K3H2PO4
E) KH2P

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following atoms of elements is most likely to form a 2- ion?
A) Potassium
B) Magnesium
C) Phosphorus
D) Bromine
E) Sulfur
A) Potassium
B) Magnesium
C) Phosphorus
D) Bromine
E) Sulfur

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is the correct formula for cobalt(III)bromide?
A) CoBr
B) CoBr3
C) Co2Br3
D) Co3Br2
E) Co3Br
A) CoBr
B) CoBr3
C) Co2Br3
D) Co3Br2
E) Co3Br

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following sets of ions is present in calcium hydrogen phosphate,CaHPO4?
A) Ca2+ and PO43-
B) Ca2+ and HPO42-
C) Ca+ and HPO4-
D) Ca3+ and HPO43-
E) Ca2+,H+,P3-,and O2-
A) Ca2+ and PO43-
B) Ca2+ and HPO42-
C) Ca+ and HPO4-
D) Ca3+ and HPO43-
E) Ca2+,H+,P3-,and O2-

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
54
The formula for aluminum fluoride is _____.
A) AlF3
B) AlF
C) Al2F
D) AlF4
E) AlF2
A) AlF3
B) AlF
C) Al2F
D) AlF4
E) AlF2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
55
The correct name for Cd2+ is _____.
A) monocadmium ion
B) cadmium(II)ion
C) cadmium(2)ion
D) cadmium(I)ion
E) cadmium
A) monocadmium ion
B) cadmium(II)ion
C) cadmium(2)ion
D) cadmium(I)ion
E) cadmium

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
56
What is the symbol for an ion of an element that has 12 protons and 10 electrons?
A) Mg2+
B) Mg2-
C) Ne2+
D) Ne2-
E) Ti2+
A) Mg2+
B) Mg2-
C) Ne2+
D) Ne2-
E) Ti2+

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
57
For a nonmetal in Group 6A of the periodic table,the most common monatomic ion will have a charge of _____.
A) 3-
B) 2-
C) 1-
D) 1+
E) 2+
A) 3-
B) 2-
C) 1-
D) 1+
E) 2+

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is the correct formula for calcium nitrate?
A) CaN
B) Ca3N2
C) CaNO2
D) Ca3(NO3)2
E) Ca(NO3)2
A) CaN
B) Ca3N2
C) CaNO2
D) Ca3(NO3)2
E) Ca(NO3)2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
59
C2H2F4 is the formula for two possible molecules.C2H2F4 is an example of a(n)_____.
A) structural formula
B) empirical formula
C) condensed formula
D) spatial formula
E) molecular formula
A) structural formula
B) empirical formula
C) condensed formula
D) spatial formula
E) molecular formula

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
60
The chemical formula for chromium(III)sulfate is _____.
A) CrSO4
B) Cr(SO4)3
C) Cr2(SO4)3
D) Cr2SO4
E) Cr3(SO4)2
A) CrSO4
B) Cr(SO4)3
C) Cr2(SO4)3
D) Cr2SO4
E) Cr3(SO4)2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
61
What is the formula for the compound that is formed of ammonium and bromide ions?
A) NH3Br
B) NH4Br
C) NH3Br2
D) NH4Br2
E) (NH4)2Br
A) NH3Br
B) NH4Br
C) NH3Br2
D) NH4Br2
E) (NH4)2Br

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following is the correct molar mass of sodium sulfate?
A) 55.06 g/mol
B) 119.1 g/mol
C) 78.05 g/mol
D) 142.0 g/mol
E) 110.0 g/mol
A) 55.06 g/mol
B) 119.1 g/mol
C) 78.05 g/mol
D) 142.0 g/mol
E) 110.0 g/mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is the correct name for the compound CCl4?
A) Carbon chlorine
B) Tetracarbon chloride
C) Carbon tetrachloride
D) Carbon(IV)chloride
E) Tetrachlorocarbide
A) Carbon chlorine
B) Tetracarbon chloride
C) Carbon tetrachloride
D) Carbon(IV)chloride
E) Tetrachlorocarbide

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
64
What mass of aluminum contains the same number of atoms as 3.0 g of lead?
A) 23 g
B) 0.014 g
C) 3.0 g
D) 0.39 g
E) 0.11 g
A) 23 g
B) 0.014 g
C) 3.0 g
D) 0.39 g
E) 0.11 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
65
A nail is coated with a 0.053 cm thick layer of zinc.The surface area of the nail is 8.59 cm2.The density of zinc is 7.13 g/cm3.How many zinc atoms are used in the coating?
A) 5.9 × 1020 atoms
B) 3.0 × 1022 atoms
C) 3.8 × 1022 atoms
D) 2.0 × 1024 atoms
E) 1.3 × 1026 atoms
A) 5.9 × 1020 atoms
B) 3.0 × 1022 atoms
C) 3.8 × 1022 atoms
D) 2.0 × 1024 atoms
E) 1.3 × 1026 atoms

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
66
The correct name for the compound P2F4 is _____.
A) diphosphorus tetrafluoride
B) phosphorus fluoride
C) diphosphide tetrafluoride
D) diphosphorus tetrafluorine
E) phosphorus tetrafluorine
A) diphosphorus tetrafluoride
B) phosphorus fluoride
C) diphosphide tetrafluoride
D) diphosphorus tetrafluorine
E) phosphorus tetrafluorine

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
67
Calculate the mass of 8.04 × 10-3 mol of O2.
A) 2.51 × 10-4 g
B) 5.03 × 10-4 g
C) 0.129 g
D) 3.89 g
E) 0.257 g
A) 2.51 × 10-4 g
B) 5.03 × 10-4 g
C) 0.129 g
D) 3.89 g
E) 0.257 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
68
Calculate the number of moles in 0.48 g of Cu.
A) 0.033 mol
B) 0.48 mol
C) 31 mol
D) 7.6 × 10−3 mol
E) 1.3 × 102 mol
A) 0.033 mol
B) 0.48 mol
C) 31 mol
D) 7.6 × 10−3 mol
E) 1.3 × 102 mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
69
The molar mass of platinum is 195.08 g/mol.What is the mass of 1.00 × 102 Pt atoms?
A) 8.51 × 10-25 g
B) 3.24 × 10-24 g
C) 1.67 × 10-22 g
D) 3.24 × 10-22 g
E) 3.24 × 10-20 g
A) 8.51 × 10-25 g
B) 3.24 × 10-24 g
C) 1.67 × 10-22 g
D) 3.24 × 10-22 g
E) 3.24 × 10-20 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
70
Calculate the mass of 0.50 mol of chromium(III)sulfide.
A) 2.5 × 10-3 g
B) 5.9 × 10-3 g
C) 42 g
D) 1.0 × 102 g
E) 110 g
A) 2.5 × 10-3 g
B) 5.9 × 10-3 g
C) 42 g
D) 1.0 × 102 g
E) 110 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
71
Calculate the number of moles of aluminum oxide in 6.83 g of Al2O3.
A) 6.70 × 10-2 mol
B) 6.96 × 102 mol
C) 0.253 mol
D) 0.127 mol
E) 1.56 × 10-3 mol
A) 6.70 × 10-2 mol
B) 6.96 × 102 mol
C) 0.253 mol
D) 0.127 mol
E) 1.56 × 10-3 mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
72
A 0.4272 g sample of an element contains 2.241 × 1021 atoms.What is the symbol of the element?
A) In
B) Cs
C) Sb
D) Xe
E) Te
A) In
B) Cs
C) Sb
D) Xe
E) Te

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is the correct molar mass of calcium chloride hexahydrate?
A) 75.53 g/mol
B) 111.0 g/mol
C) 117.0 g/mol
D) 183.6 g/mol
E) 219.1 g/mol
A) 75.53 g/mol
B) 111.0 g/mol
C) 117.0 g/mol
D) 183.6 g/mol
E) 219.1 g/mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following ionic compounds has the highest melting point?
A) KBr
B) MgO
C) RbI
D) CaBr2
E) CsCl
A) KBr
B) MgO
C) RbI
D) CaBr2
E) CsCl

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
75
What is the molecular mass of cyclononane,C9H18?
A) 13.02 g/mol
B) 1963.92 g/mol
C) 109.11 g/mol
D) 126.24 g/mol
E) 30.15 g/mol
A) 13.02 g/mol
B) 1963.92 g/mol
C) 109.11 g/mol
D) 126.24 g/mol
E) 30.15 g/mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is the common name for the compound PH3?
A) Laughing gas
B) Hydrazine
C) Nitroglycerin
D) Ammonia
E) Phosphine
A) Laughing gas
B) Hydrazine
C) Nitroglycerin
D) Ammonia
E) Phosphine

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
77
A 0.0050 g sample of boron contains _____ boron atoms.
A) 4.6 × 10-4
B) 7.7 × 10-28
C) 2.8 × 1020
D) 3.1 × 1021
E) 3.3 × 1022
A) 4.6 × 10-4
B) 7.7 × 10-28
C) 2.8 × 1020
D) 3.1 × 1021
E) 3.3 × 1022

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
78
What is the approximate mass of 0.71 mol Na?
A) 1.2 × 10-24 g
B) 12 g
C) 16 g
D) 0.031 g
E) 32 g
A) 1.2 × 10-24 g
B) 12 g
C) 16 g
D) 0.031 g
E) 32 g

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is the correct name for the compound SrCl2?
A) Strontium dichloride
B) Strontium dichlorine
C) Strontium(III)dichloride
D) Strontium chloride
E) Iodine strontide
A) Strontium dichloride
B) Strontium dichlorine
C) Strontium(III)dichloride
D) Strontium chloride
E) Iodine strontide

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
80
You have 2.50 g of each of the following elements: Ca,Cu,Cs,C,and Cr.Which sample contains the largest number of atoms?
A) Ca
B) Cu
C) Cs
D) C
E) Cr
A) Ca
B) Cu
C) Cs
D) C
E) Cr

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck


