Exam 3: Atoms, molecules, and Ions
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: The Tools of Quantitative Chemistry67 Questions
Exam 3: Atoms, molecules, and Ions101 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions65 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure89 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals63 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids69 Questions
Exam 13: The Solid State62 Questions
Exam 14: Solutions and Their Behavior79 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions72 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria77 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases95 Questions
Exam 17: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria86 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy66 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions86 Questions
Exam 21: Environmental Chemistry: Earths Environment, energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements82 Questions
Exam 23: The Chemistry of the Transition Elements79 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry48 Questions
Exam 26: Nuclear Chemistry190 Questions
Select questions type
Which of the following statements is not true about the element vanadium?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
D
If two different isotopes have the same atomic number,it means that _____.
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
C
Which of the following formulas is not correct?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
D
The masses of isotopes and their abundances are determined experimentally using _____.
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following is the correct atomic symbol for an element with 17 protons and 18 neutrons?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following atoms of elements is most likely to form a 2- ion?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following is the correct formula for an ionic compound that contains barium ions and carbonate ions?
(Multiple Choice)
4.8/5  (37)
(37)
If 1.00 g of an unknown molecular compound contains 8.35 × 1021 molecules,what is its molar mass?
(Multiple Choice)
4.8/5  (32)
(32)
An ionic compound has the formula MCl2.The mass of 0.3011 mol of the compound is 62.69 grams.What is the identity of the metal?
(Multiple Choice)
4.7/5  (28)
(28)
The fully hydrated form of sodium sulfate is the decahydrate,Na2SO4⋅10H2O.This compound dehydrates (loses some waters of hydration)when heated.A sample of partially dehydrated sodium sulfate was found to have a molar mass of 232 g/mol.How many water molecules are found per formula unit in in this sample? (Determine n in Na2SO4⋅ nH2O).
(Multiple Choice)
4.8/5  (45)
(45)
A nail is coated with a 0.053 cm thick layer of zinc.The surface area of the nail is 8.59 cm2.The density of zinc is 7.13 g/cm3.How many zinc atoms are used in the coating?
(Multiple Choice)
4.9/5  (37)
(37)
The numerical quantity of a mole,6.022 × 1023,is defined as the number of atoms in a specific mass of an element.What is the mass and the identity of the element used to define one mole?
(Essay)
4.8/5  (33)
(33)
How many protons,neutrons,and electrons are present in an atom of iron-59?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following atomic symbols represents an isotope of 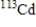 ?
?
(Multiple Choice)
4.8/5  (34)
(34)
Oxygen and _____ are the two most abundant elements in the Earth's crust.
(Short Answer)
4.8/5  (42)
(42)
Showing 1 - 20 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)