Deck 16: Aqueous Ionic Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/173
Play
Full screen (f)
Deck 16: Aqueous Ionic Equilibrium
1
Calculate the pH of a solution formed by mixing 250.0 mL of 0.15 mol L-1 NH4Cl with 200.0 mL of 0.12 mol L-1 NH3. The Kb for NH3 is 1.8 × 10-5.
A) 9.06
B) 9.45
C) 4.55
D) 4.74
E) 9.26
A) 9.06
B) 9.45
C) 4.55
D) 4.74
E) 9.26
9.06
2
Calculate the pH of a solution formed by mixing 200.0 mL of 0.30 mol L-1 HClO with 100.0 mL of 0.20 mol L-1 KClO. The Ka for HClO is 2.9 × 10-8.
A) 5.99
B) 8.01
C) 7.54
D) 7.06
E) 6.46
A) 5.99
B) 8.01
C) 7.54
D) 7.06
E) 6.46
7.06
3
If the pKa of HCOOH is 3.74 and the pH of an HCOOH/HCOONa buffer solution is 3.74, which of the following is TRUE?
A) [HCOOH] > [HCOONa]
B) [HCOOH] = [HCOONa]
C) [HCOOH] < [HCOONa]
D) [HCOOH] << [HCOONa]
E) [HCOOH] >> [HCOONa]
A) [HCOOH] > [HCOONa]
B) [HCOOH] = [HCOONa]
C) [HCOOH] < [HCOONa]
D) [HCOOH] << [HCOONa]
E) [HCOOH] >> [HCOONa]
[HCOOH] = [HCOONa]
4
Calculate the pH of a buffer that is 0.058 mol L-1 HF and 0.058 mol L-1 LiF. The Ka for HF is 3.5 × 10-4.
A) 2.86
B) 9.31
C) 10.54
D) 3.46
E) 4.69
A) 2.86
B) 9.31
C) 10.54
D) 3.46
E) 4.69

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
5
Calculate the pH of a buffer that is 0.105 mol L-1 CH3COOH and 0.146 mol L-1 CH3COOK. The Ka for CH3COOH is 1.8 × 10-5.
A) 4.89
B) 9.11
C) 4.74
D) 9.26
E) 4.60
A) 4.89
B) 9.11
C) 4.74
D) 9.26
E) 4.60

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
6
Calculate the pH of a solution formed by mixing 100.0 mL of 0.20 mol L-1 HClO with 200.0 mL of 0.30 mol L-1 KClO. The Ka for HClO is 2.9 × 10-8.
A) 5.99
B) 8.01
C) 7.54
D) 7.06
E) 6.46
A) 5.99
B) 8.01
C) 7.54
D) 7.06
E) 6.46

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
7
Calculate the pH of a buffer that is 0.040 mol L-1 HF and 0.020 mol L-1 LiF. The Ka for HF is 3.5 × 10-4.
A) 2.06
B) 4.86
C) 3.15
D) 3.46
E) 3.76
A) 2.06
B) 4.86
C) 3.15
D) 3.46
E) 3.76

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
8
Which one of the following statements is TRUE?
A) A buffer is an aqueous solution composed of two weak acids.
B) A buffer can absorb an unlimited amount of acid or base.
C) A buffer resists pH change by neutralizing added acids and bases.
D) A buffer does not change pH when large amounts of a strong acid or a strong base are added.
E) The common ion in a buffer solution does not have any effect on the dissociation of the acidic component of the buffer solution.
A) A buffer is an aqueous solution composed of two weak acids.
B) A buffer can absorb an unlimited amount of acid or base.
C) A buffer resists pH change by neutralizing added acids and bases.
D) A buffer does not change pH when large amounts of a strong acid or a strong base are added.
E) The common ion in a buffer solution does not have any effect on the dissociation of the acidic component of the buffer solution.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
9
Calculate the pH of a buffer that is 0.225 mol L-1 CH3COOH and 0.162 mol L-1 CH3COOK. The Ka for CH3COOH is 1.8 × 10-5.
A) 4.89
B) 9.11
C) 4.74
D) 9.26
E) 4.60
A) 4.89
B) 9.11
C) 4.74
D) 9.26
E) 4.60

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
10
If the pKa of HCOOH is 3.74 and the pH of an HCOOH/HCOONa solution is 3.11, which of the following is TRUE?
A) [HCOOH] < [HCOONa]
B) [HCOOH] = [HCOONa]
C) [HCOOH] << [HCOONa]
D) [HCOOH] > [HCOONa]
E) [HCOOH] >> [HCOONa]
A) [HCOOH] < [HCOONa]
B) [HCOOH] = [HCOONa]
C) [HCOOH] << [HCOONa]
D) [HCOOH] > [HCOONa]
E) [HCOOH] >> [HCOONa]

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
11
Calculate the pH of a buffer that is 0.020 mol L-1 HF and 0.040 mol L-1 LiF. The Ka for HF is 3.5 × 10-4.
A) 2.06
B) 4.86
C) 3.16
D) 3.46
E) 3.76
A) 2.06
B) 4.86
C) 3.16
D) 3.46
E) 3.76

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
12
You wish to prepare a buffer containing CH3COOH with a pH of 5.44. If the pKa of acetic acid is 4.74, what ratio of [CH3COO⁻]/[CH3COOH] must you use?
A) 0.70
B) 0.20
C) 1.4
D) 5.0
E) 1.1
A) 0.70
B) 0.20
C) 1.4
D) 5.0
E) 1.1

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
13
Calculate the pH of a buffer that is 0.158 mol L-1 HClO and 0.099 mol L-1 NaClO. The Ka for HClO is 2.9 × 10-8.
A) 7.54
B) 6.67
C) 3.77
D) 6.46
E) 7.33
A) 7.54
B) 6.67
C) 3.77
D) 6.46
E) 7.33

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
14
You wish to prepare a buffer containing CH3COOH with a pH of 4.24. If the pKa of acetic acid is 4.74, what ratio of [CH3COO⁻]/[CH3COOH] must you use?
A) 0.10
B) 0.50
C) 0.32
D) 2.0
E) 2.8
A) 0.10
B) 0.50
C) 0.32
D) 2.0
E) 2.8

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
15
Identify a good buffer.
A) a solution containing small amounts of both a weak acid and its conjugate base
B) a solution containing significant amounts of both a strong acid and a strong base
C) a solution containing small amounts of both a strong acid and a strong base
D) a solution containing significant amounts of both a weak acid and a strong acid
E) a solution containing significant amounts of both a weak acid and its conjugate base
A) a solution containing small amounts of both a weak acid and its conjugate base
B) a solution containing significant amounts of both a strong acid and a strong base
C) a solution containing small amounts of both a strong acid and a strong base
D) a solution containing significant amounts of both a weak acid and a strong acid
E) a solution containing significant amounts of both a weak acid and its conjugate base

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
16
Calculate the pH of a solution formed by mixing 250.0 mL of 0.15 mol L-1 NH4Cl with 100.0 mL of 0.20 mol L-1 NH3. The Kb for NH3 is 1.8 × 10-5.
A) 9.13
B) 9.25
C) 9.53
D) 4.74
E) 8.98
A) 9.13
B) 9.25
C) 9.53
D) 4.74
E) 8.98

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following solutions represents a good buffer system?
A) a solution that is 0.10 mol L-1 CH3COOH and 0.10 mol L-1 CH3COOLi
B) a solution that is 0.10 mol L-1 HF and 0.10 mol L-1 CH3COONa
C) a solution that is 0.10 mol L-1 HCl and 0.10 mol L-1 NH4+
D) a solution that is 0.10 mol L-1 NaOH and 0.10 mol L-1 KOH
E) a solution that is 0.10 mol L-1 HCl and 0.10 mol L-1 KCl
A) a solution that is 0.10 mol L-1 CH3COOH and 0.10 mol L-1 CH3COOLi
B) a solution that is 0.10 mol L-1 HF and 0.10 mol L-1 CH3COONa
C) a solution that is 0.10 mol L-1 HCl and 0.10 mol L-1 NH4+
D) a solution that is 0.10 mol L-1 NaOH and 0.10 mol L-1 KOH
E) a solution that is 0.10 mol L-1 HCl and 0.10 mol L-1 KCl

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
18
A buffer solution is 0.100 mol L-1 in both C6H5COOH and C6H5COOLi and has a pH of 4.19. Which of the following pH values would you expect after the addition of a small amount of a strong base?
A) 3.89
B) 3.69
C) 5.69
D) 4.49
E) 8.89
A) 3.89
B) 3.69
C) 5.69
D) 4.49
E) 8.89

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following solutions is a good buffer system?
A) a solution that is 0.10 mol L-1 NaCl and 0.10 mol L-1 HCl
B) a solution that is 0.10 mol L-1 HCN and 0.10 mol L-1 LiCN
C) a solution that is 0.10 mol L-1 NaOH and 0.10 mol L-1 HNO3
D) a solution that is 0.10 mol L-1 HNO3 and 0.10 mol L-1 KNO3
E) a solution that is 0.10 mol L-1 HCN and 0.10 mol L-1 NaCl
A) a solution that is 0.10 mol L-1 NaCl and 0.10 mol L-1 HCl
B) a solution that is 0.10 mol L-1 HCN and 0.10 mol L-1 LiCN
C) a solution that is 0.10 mol L-1 NaOH and 0.10 mol L-1 HNO3
D) a solution that is 0.10 mol L-1 HNO3 and 0.10 mol L-1 KNO3
E) a solution that is 0.10 mol L-1 HCN and 0.10 mol L-1 NaCl

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
20
If the pKa of HCOOH is 3.74 and the pH of an HCOOH/HCOONa buffer solution is 3.89, which of the following is TRUE?
A) [HCOOH] < [HCOONa]
B) [HCOOH] = [HCOONa]
C) [HCOOH] > [HCOONa]
D) [HCOOH] >> [HCOONa]
E) [HCOOH] << [HCOONa]
A) [HCOOH] < [HCOONa]
B) [HCOOH] = [HCOONa]
C) [HCOOH] > [HCOONa]
D) [HCOOH] >> [HCOONa]
E) [HCOOH] << [HCOONa]

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
21
Define buffer capacity.
A) Buffer capacity is the amount of acid or base that can be added to a buffer without destroying its effectiveness.
B) Buffer capacity is the amount of acid that can be added until all the base in the buffer is consumed.
C) Buffer capacity is the amount of base that can be added until all the acid in the buffer is consumed.
D) Buffer capacity is the amount of acid that can be added until all the acid in the buffer is consumed.
E) Buffer capacity is the amount of base that can be added until all the base in the buffer is consumed.
A) Buffer capacity is the amount of acid or base that can be added to a buffer without destroying its effectiveness.
B) Buffer capacity is the amount of acid that can be added until all the base in the buffer is consumed.
C) Buffer capacity is the amount of base that can be added until all the acid in the buffer is consumed.
D) Buffer capacity is the amount of acid that can be added until all the acid in the buffer is consumed.
E) Buffer capacity is the amount of base that can be added until all the base in the buffer is consumed.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
22
A 1.00 L buffer solution is 0.250 mol L-1 in HF and 0.250 mol L-1 in LiF. Calculate the pH of the solution after the addition of 0.150 moles of solid LiOH. Assume no volume change upon the addition of the base. The Ka for HF is 3.5 × 10-4.
A) 3.46
B) 4.06
C) 2.85
D) 3.63
E) 4.24
A) 3.46
B) 4.06
C) 2.85
D) 3.63
E) 4.24

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
23
Calculate the mole ratio of HCOONa to HCOOH required to make a buffer with pH of 3.62. Ka of HCOOH is 1.8 × 10-4.
A) 0.122
B) 3.00
C) 0.75
D) 1.22
E) 10.0
A) 0.122
B) 3.00
C) 0.75
D) 1.22
E) 10.0

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following acids (listed with pKa values) and its conjugate would be the best choice to make a buffer with a pH of 10.40?
A) HC7H5O2, pKa = 4.19
B) HF, pKa = 3.46
C) HClO, pKa = 7.54
D) HCN, pKa = 9.31
E) HClO2, pKa = 1.96
A) HC7H5O2, pKa = 4.19
B) HF, pKa = 3.46
C) HClO, pKa = 7.54
D) HCN, pKa = 9.31
E) HClO2, pKa = 1.96

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following acids (listed with pKa values) and its conjugate would be the best choice to make a buffer with a pH of 5.20?
A) HC7H5O2, pKa = 4.19
B) HF, pKa = 3.46
C) HClO, pKa = 7.54
D) HCN, pKa = 9.31
E) HClO2, pKa = 1.96
A) HC7H5O2, pKa = 4.19
B) HF, pKa = 3.46
C) HClO, pKa = 7.54
D) HCN, pKa = 9.31
E) HClO2, pKa = 1.96

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following acids (listed with Ka values) and their conjugate base would be the best choice to make a buffer with a pH of 8.10?
A) C6H5COOH, Ka = 6.5 × 10-5
B) HF, Ka = 3.5 × 10-4
C) HClO, Ka = 2.9 × 10-8
D) HCN, Ka = 4.9 × 10-10
E) HClO2, Ka = 1.1 × 10-2
A) C6H5COOH, Ka = 6.5 × 10-5
B) HF, Ka = 3.5 × 10-4
C) HClO, Ka = 2.9 × 10-8
D) HCN, Ka = 4.9 × 10-10
E) HClO2, Ka = 1.1 × 10-2

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following acids (listed with pKa values) and its conjugate would be the best choice to make a buffer with a pH of 2.12?
A) HC7H5O2, pKa = 4.19
B) CH3COOH, pKa = 4.74
C) HClO, pKa = 7.54
D) HCN, pKa = 9.31
E) HClO2, pKa = 1.96
A) HC7H5O2, pKa = 4.19
B) CH3COOH, pKa = 4.74
C) HClO, pKa = 7.54
D) HCN, pKa = 9.31
E) HClO2, pKa = 1.96

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following acids (listed with Ka values) and their conjugate base would be the best choice to make a buffer with a pH of 10.40?
A) C6H5COOH, Ka = 6.5 × 10-5
B) HF, Ka = 3.5 × 10-4
C) HClO, Ka = 2.9 × 10-8
D) HCN, Ka = 4.9 × 10-10
E) HClO2, Ka = 1.1 × 10-2
A) C6H5COOH, Ka = 6.5 × 10-5
B) HF, Ka = 3.5 × 10-4
C) HClO, Ka = 2.9 × 10-8
D) HCN, Ka = 4.9 × 10-10
E) HClO2, Ka = 1.1 × 10-2

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is TRUE?
A) An effective buffer has a [base]/[acid] ratio in the range of 10-100.
B) A buffer is most resistant to pH change when [acid] = [conjugate base].
C) An effective buffer has very small absolute concentrations of acid and conjugate base.
D) The capacity of a buffer cannot be exceeded by adding too much strong base. It can only be exceeded by adding too much strong acid.
E) A buffer has the best resistance to pH change when the buffer's pH is significantly lower than the pKa of the acid used to make the buffer solution.
A) An effective buffer has a [base]/[acid] ratio in the range of 10-100.
B) A buffer is most resistant to pH change when [acid] = [conjugate base].
C) An effective buffer has very small absolute concentrations of acid and conjugate base.
D) The capacity of a buffer cannot be exceeded by adding too much strong base. It can only be exceeded by adding too much strong acid.
E) A buffer has the best resistance to pH change when the buffer's pH is significantly lower than the pKa of the acid used to make the buffer solution.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
30
A 1.50 L buffer solution is 0.250 mol L-1 in HF and 0.250 mol L-1 in NaF. Calculate the pH of the solution after the addition of 0.0500 moles of solid NaOH. Assume no volume change upon the addition of the base. The Ka for HF is 3.5 × 10-4.
A) 3.34
B) 3.46
C) 3.57
D) 3.63
E) 2.89
A) 3.34
B) 3.46
C) 3.57
D) 3.63
E) 2.89

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
31
A 1.00 L buffer solution is 0.250 mol L-1 in HF and 0.250 mol L-1 in NaF. Calculate the pH of the solution after the addition of 100.0 mL of 1.00 mol L-1 HCl. The Ka for HF is 3.5 × 10-4.
A) 3.09
B) 4.11
C) 3.82
D) 3.46
E) 2.78
A) 3.09
B) 4.11
C) 3.82
D) 3.46
E) 2.78

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the pH of a solution formed by mixing 250.0 mL of 0.15 mol L-1 HCOOH with 100.0 mL of 0.20 mol L-1 HCOOLi. The Ka for HCHO2 is 1.8 × 10-4.
A) 3.87
B) 3.74
C) 10.53
D) 3.47
E) 10.13
A) 3.87
B) 3.74
C) 10.53
D) 3.47
E) 10.13

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
33
Calculate the pH of a solution formed by mixing 200.0 mL of 0.30 mol L-1 HClO with 300.0 mL of 0.20 mol L-1 KClO. The Ka for HClO is 2.9 × 10-8.
A) 5.99
B) 8.01
C) 7.54
D) 7.06
E) 6.46
A) 5.99
B) 8.01
C) 7.54
D) 7.06
E) 6.46

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following acids (listed with Ka values) and their conjugate base would be the best choice to make a buffer with a pH of 2.34?
A) C6H5COOH, Ka = 6.5 × 10-5
B) HF, Ka = 3.5 × 10-4
C) HClO, Ka = 2.9 × 10-8
D) HCN, Ka = 4.9 × 10-10
E) HClO2, Ka = 1.1 × 10-2
A) C6H5COOH, Ka = 6.5 × 10-5
B) HF, Ka = 3.5 × 10-4
C) HClO, Ka = 2.9 × 10-8
D) HCN, Ka = 4.9 × 10-10
E) HClO2, Ka = 1.1 × 10-2

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following acids (listed with Ka values) and their conjugate base would be the best choice to make a buffer with a pH of 5.20?
A) C6H5COOH, Ka = 6.5 × 10-5
B) HF, Ka = 3.5 × 10-4
C) HClO, Ka = 2.9 × 10-8
D) HCN, Ka = 4.9 × 10-10
E) HClO2, Ka = 1.1 × 10-2
A) C6H5COOH, Ka = 6.5 × 10-5
B) HF, Ka = 3.5 × 10-4
C) HClO, Ka = 2.9 × 10-8
D) HCN, Ka = 4.9 × 10-10
E) HClO2, Ka = 1.1 × 10-2

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
36
What is the maximum ratio of conjugate base to acid in an effective buffer?
A) 2
B) 5
C) 10
D) 20
E) 15
A) 2
B) 5
C) 10
D) 20
E) 15

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following acids (listed with pKa values) and its conjugate would be the best choice to make a buffer with a pH of 8.10?
A) HC7H5O2, pKa = 4.19
B) HF, pKa = 3.46
C) HClO, pKa = 7.54
D) HCN, pKa = 9.31
E) HClO2, pKa = 1.96
A) HC7H5O2, pKa = 4.19
B) HF, pKa = 3.46
C) HClO, pKa = 7.54
D) HCN, pKa = 9.31
E) HClO2, pKa = 1.96

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
38
A 1.00 L buffer solution is 0.150 mol L-1 in C6H5COOH and 0.250 mol L-1 in C6H5COOLi. Calculate the pH of the solution after the addition of 100.0 mL of 1.00 mol L-1 HCl. The Ka for HC7H5O2 is 6.5 × 10-5.
A) 4.19
B) 5.03
C) 4.41
D) 3.34
E) 3.97
A) 4.19
B) 5.03
C) 4.41
D) 3.34
E) 3.97

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
39
Calculate the mole ratio of CH3COONa to CH3COOH required to make a buffer with pH of 4.83. Ka of CH3COOH is 1.8 × 10-5.
A) 0.122
B) 3.00
C) 0.75
D) 1.22
E) 10.0
A) 0.122
B) 3.00
C) 0.75
D) 1.22
E) 10.0

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
40
Calculate the pH of a solution formed by mixing 150.0 mL of 0.10 mol L-1 C6H5COOH with 100.0 mL of 0.30 mol L-1 C6H5COONa. The Ka for C6H5COOH is 6.5 × 10-5.
A) 4.19
B) 9.69
C) 4.49
D) 4.31
E) 10.51
A) 4.19
B) 9.69
C) 4.49
D) 4.31
E) 10.51

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
41
The titration of 80.0 mL of an unknown concentration H3PO4 solution requires 126 mL of 0.218 mol L-1 KOH solution. What is the concentration of the H3PO4 solution (in mol L-1)?
A) 1.03 mol L-1
B) 0.343 mol L-1
C) 0.114 mol L-1
D) 0.138 mol L-1
E) 0.0461 mol L-1
A) 1.03 mol L-1
B) 0.343 mol L-1
C) 0.114 mol L-1
D) 0.138 mol L-1
E) 0.0461 mol L-1

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
42
A 100.0 mL sample of 0.18 mol L-1 HClO4 is titrated with 0.27 mol L-1 NaOH. Determine the pH of the solution after the addition of 30.0 mL of NaOH.
A) 0.86
B) 1.21
C) 2.00
D) 1.12
E) 2.86
A) 0.86
B) 1.21
C) 2.00
D) 1.12
E) 2.86

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
43
A 100.0 mL sample of 0.180 mol L-1 HClO4 is titrated with 0.270 mol L-1 NaOH. Determine the pH of the solution after the addition of 75.0 mL of NaOH.
A) 12.1
B) 2.65
C) 11.35
D) 1.89
E) 13.06
A) 12.1
B) 2.65
C) 11.35
D) 1.89
E) 13.06

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
44
A 1.0 L buffer solution is 0.250 mol L-1 CH3COOH and 0.050 mol L-1 CH3COOLi. Which of the following actions will exceed the capacity of the buffer?
A) adding 0.050 moles of CH3COOLi
B) adding 0.050 moles of CH3COOH
C) adding 0.050 moles of HCl
D) adding 0.050 moles of NaCl
E) adding 0.050 moles of NH4Cl
A) adding 0.050 moles of CH3COOLi
B) adding 0.050 moles of CH3COOH
C) adding 0.050 moles of HCl
D) adding 0.050 moles of NaCl
E) adding 0.050 moles of NH4Cl

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
45
Identify the indicator that can be used at the lowest pH.
A) alizarin
B) thymol blue
C) crystal violet
D) phenolphthalein
E) alizarin yellow R
A) alizarin
B) thymol blue
C) crystal violet
D) phenolphthalein
E) alizarin yellow R

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
46
Identify the pH of normal blood.
A) 7.0
B) 7.2
C) 7.4
D) 7.6
E) 7.8
A) 7.0
B) 7.2
C) 7.4
D) 7.6
E) 7.8

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
47
Calculate the mole ratio of C6H5COONa to C6H5COOH required to make a buffer with pH of 4.68. Ka of C6H5COOH is 6.3 × 10-5.
A) 0.122
B) 3.02
C) 0.75
D) 1.22
E) 10.0
A) 0.122
B) 3.02
C) 0.75
D) 1.22
E) 10.0

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
48
A 100.0 mL sample of 0.18 mol L-1 HClO4 is titrated with 0.27 mol L-1 NaOH. Determine the pH of the solution after the addition of 66.67 mL of NaOH (this is the equivalence point).
A) 0.97
B) 13.03
C) 2.76
D) 11.24
E) 7.00
A) 0.97
B) 13.03
C) 2.76
D) 11.24
E) 7.00

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
49
When titrating a strong monoprotic acid with KOH at 25 °C, the ________.
A) pH will be less than 7 at the equivalence point
B) pH will be greater than 7 at the equivalence point
C) titration will require more moles of base than acid to reach the equivalence point
D) pH will be equal to 7 at the equivalence point
E) titration will require more moles of acid than base to reach the equivalence point
A) pH will be less than 7 at the equivalence point
B) pH will be greater than 7 at the equivalence point
C) titration will require more moles of base than acid to reach the equivalence point
D) pH will be equal to 7 at the equivalence point
E) titration will require more moles of acid than base to reach the equivalence point

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
50
Identify the indicator that can be used at the highest pH.
A) alizarin
B) thymol blue
C) crystal violet
D) phenolphthalein
E) alizarin yellow R
A) alizarin
B) thymol blue
C) crystal violet
D) phenolphthalein
E) alizarin yellow R

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
51
Identify the indicator that has two endpoints.
A) phenol red
B) thymol blue
C) crystal violet
D) phenolphthalein
E) alizarin yellow R
A) phenol red
B) thymol blue
C) crystal violet
D) phenolphthalein
E) alizarin yellow R

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
52
When titrating a monoprotic strong acid with a weak base at 25 °C, the ________.
A) pH will be 7 at the equivalence point
B) pH will be greater than 7 at the equivalence point
C) titration will require more moles of base than acid to reach the equivalence point
D) titration will require more moles of acid than base to reach the equivalence point
E) pH will be less than 7 at the equivalence point
A) pH will be 7 at the equivalence point
B) pH will be greater than 7 at the equivalence point
C) titration will require more moles of base than acid to reach the equivalence point
D) titration will require more moles of acid than base to reach the equivalence point
E) pH will be less than 7 at the equivalence point

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
53
The titration of 25.0 mL of an unknown concentration H2SO4 solution requires 83.6 mL of a 0.12 mol L-1 LiOH solution. What is the concentration of the H2SO4 solution (in mol L-1)?
A) 0.20 mol L-1
B) 0.40 mol L-1
C) 0.10 mol L-1
D) 0.36 mol L-1
E) 0.25 mol L-1
A) 0.20 mol L-1
B) 0.40 mol L-1
C) 0.10 mol L-1
D) 0.36 mol L-1
E) 0.25 mol L-1

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is TRUE?
A) The equivalence point is where the amount of acid equals the amount of base during any acid-base titration.
B) At the equivalence point, the pH is always 7.
C) An indicator is not pH sensitive.
D) A titration curve is a plot of pH vs. the [base]/[acid] ratio.
E) A titration curve is a plot of pH versus titration time.
A) The equivalence point is where the amount of acid equals the amount of base during any acid-base titration.
B) At the equivalence point, the pH is always 7.
C) An indicator is not pH sensitive.
D) A titration curve is a plot of pH vs. the [base]/[acid] ratio.
E) A titration curve is a plot of pH versus titration time.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
55
A 100.0 mL sample of 0.18 mol L-1 HClO4 is titrated with 0.27 mol L-1 NaOH. Determine the pH of the solution after the addition of 100.0 mL of NaOH.
A) 13.1
B) 12.7
C) 1.3
D) 0.9
E) 12.9
A) 13.1
B) 12.7
C) 1.3
D) 0.9
E) 12.9

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
56
Identify the most commonly used indicator among the following five.
A) alizarin
B) thymol blue
C) crystal violet
D) phenolphthalein
E) alizarin yellow R
A) alizarin
B) thymol blue
C) crystal violet
D) phenolphthalein
E) alizarin yellow R

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
57
When titrating a weak monoprotic acid with NaOH at 25 °C, the ________.
A) pH will be less than 7 at the equivalence point
B) pH will be equal to 7 at the equivalence point
C) pH will be greater than 7 at the equivalence point
D) titration will require more moles of base than acid to reach the equivalence point
E) titration will require more moles of acid than base to reach the equivalence point
A) pH will be less than 7 at the equivalence point
B) pH will be equal to 7 at the equivalence point
C) pH will be greater than 7 at the equivalence point
D) titration will require more moles of base than acid to reach the equivalence point
E) titration will require more moles of acid than base to reach the equivalence point

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
58
What is the pH of a solution made by mixing 40.00 mL of 0.100 mol L-1 HCl with 25.00 mL of 0.100 mol L-1 KOH? Assume that the volumes of the solutions are additive.
A) 0.64
B) 1.64
C) 12.36
D) 13.36
E) 10.00
A) 0.64
B) 1.64
C) 12.36
D) 13.36
E) 10.00

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
59
A 1.0 L buffer solution is 0.050 mol L-1 CH3COOH and 0.250 mol L-1 CH3COOLi. Which of the following actions will destroy the buffer?
A) adding 0.050 moles of NaOH
B) adding 0.050 moles of HCl
C) adding 0.050 moles of CH3COOH
D) adding 0.050 moles of CH3COOLi
E) adding 0.050 moles of NH4Cl.
A) adding 0.050 moles of NaOH
B) adding 0.050 moles of HCl
C) adding 0.050 moles of CH3COOH
D) adding 0.050 moles of CH3COOLi
E) adding 0.050 moles of NH4Cl.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
60
A 100.0 mL sample of 0.18 mol L-1 HClO4 is titrated with 0.27 mol L-1 NaOH. Determine the pH of the solution before the addition of any NaOH.
A) 1.74
B) 1.05
C) 0.74
D) 0.57
E) 1.57
A) 1.74
B) 1.05
C) 0.74
D) 0.57
E) 1.57

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
61
An acid solution is 0.100 mol L-1 in HCl and 0.200 mol L-1 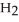
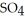 . What volume of a 0.150 mol L-1 KOH solution would completely neutralize 500.0 mL of this solution?
. What volume of a 0.150 mol L-1 KOH solution would completely neutralize 500.0 mL of this solution?
A) 0.700 L
B) 1.98 L
C) 1.67 L
D) 1.02 L
E) 0.250 L
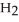
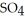 . What volume of a 0.150 mol L-1 KOH solution would completely neutralize 500.0 mL of this solution?
. What volume of a 0.150 mol L-1 KOH solution would completely neutralize 500.0 mL of this solution?A) 0.700 L
B) 1.98 L
C) 1.67 L
D) 1.02 L
E) 0.250 L

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
62
A 100.0 mL sample of 0.20 mol L-1 HF is titrated with 0.10 mol L-1 KOH. Determine the pH of the solution after the addition of 200.0 mL of KOH. The Ka of HF is 3.5 × 10-4.
A) 9.62
B) 7.00
C) 3.46
D) 10.54
E) 8.14
A) 9.62
B) 7.00
C) 3.46
D) 10.54
E) 8.14

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
63
A 100.0 mL sample of 0.20 mol L-1 HF is titrated with 0.10 mol L-1 KOH. Determine the pH of the solution before the addition of any KOH. The Ka of HF is 3.5 × 10-4.
A) 4.15
B) 0.70
C) 2.08
D) 3.46
E) 1.00
A) 4.15
B) 0.70
C) 2.08
D) 3.46
E) 1.00

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
64
Identify the salts that are in hard water.
A) CaCO3 and MgCO3
B) MgSO4 and CaSO4
C) NaCl and KBr
D) NaHSO4 and Na2SO4
E) NaOCl and HOCl
A) CaCO3 and MgCO3
B) MgSO4 and CaSO4
C) NaCl and KBr
D) NaHSO4 and Na2SO4
E) NaOCl and HOCl

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following compounds will have the highest molar solubility in pure water?
A) PbSO4, Ksp = 1.82 × 10-8
B) MgCO3, Ksp = 6.82 × 10-6
C) AgI, Ksp = 8.51 × 10-17
D) PbS, Ksp = 9.04 × 10-29
E) FeS, Ksp = 3.72 × 10-19
A) PbSO4, Ksp = 1.82 × 10-8
B) MgCO3, Ksp = 6.82 × 10-6
C) AgI, Ksp = 8.51 × 10-17
D) PbS, Ksp = 9.04 × 10-29
E) FeS, Ksp = 3.72 × 10-19

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
66
A 100.0 mL sample of 0.20 mol L-1 HF is titrated with 0.10 mol L-1 KOH. Determine the pH of the solution after the addition of 400.0 mL of KOH. The Ka of HF is 3.5 × 10-4.
A) 13.08
B) 12.60
C) 13.85
D) 12.30
E) 12.78
A) 13.08
B) 12.60
C) 13.85
D) 12.30
E) 12.78

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
67
A 100.0 mL sample of 0.10 mol L-1 NH3 is titrated with 0.10 mol L-1 HNO3. Determine the pH of the solution after the addition of 200.0 mL of HNO3. The Kb of NH3 is 1.8 × 10-5.
A) 6.44
B) 1.48
C) 2.00
D) 12.52
E) 12.00
A) 6.44
B) 1.48
C) 2.00
D) 12.52
E) 12.00

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
68
A 100.0 mL sample of 0.10 mol L-1 NH3 is titrated with 0.10 mol L-1 HNO3. Determine the pH of the solution after the addition of 50.0 mL of HNO3. The Kb of NH3 is 1.8 × 10-5.
A) 4.74
B) 7.78
C) 7.05
D) 9.26
E) 10.34
A) 4.74
B) 7.78
C) 7.05
D) 9.26
E) 10.34

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
69
A 100.0 mL sample of 0.10 mol L-1 Ca(OH)2 is titrated with 0.10 mol L-1 HBr. Determine the pH of the solution after the addition of 400.0 mL HBr.
A) 1.00
B) 1.40
C) 1.22
D) 1.30
E) 2.11
A) 1.00
B) 1.40
C) 1.22
D) 1.30
E) 2.11

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
70
A 100.0 mL sample of 0.10 mol L-1 NH3 is titrated with 0.10 mol L-1 HNO3. Determine the pH of the solution after the addition of 150.0 mL of HNO3. The Kb of NH3 is 1.8 × 10-5.
A) 1.7
B) 6.4
C) 7.6
D) 12.3
E) 2.3
A) 1.7
B) 6.4
C) 7.6
D) 12.3
E) 2.3

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
71
A 100.0 mL sample of 0.20 mol L-1 HF is titrated with 0.10 mol L-1 KOH. Determine the pH of the solution after the addition of 100.0 mL of KOH. The Ka of HF is 3.5 × 10-4.
A) 2.08
B) 3.15
C) 4.33
D) 3.46
E) 4.15
A) 2.08
B) 3.15
C) 4.33
D) 3.46
E) 4.15

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
72
A 100.0 mL sample of 0.10 mol L-1 NH3 is titrated with 0.10 mol L-1 HNO3. Determine the pH of the solution after the addition of 100.0 mL of HNO3. The Kb of NH3 is 1.8 × 10-5.
A) 6.58
B) 10.56
C) 8.72
D) 3.44
E) 5.28
A) 6.58
B) 10.56
C) 8.72
D) 3.44
E) 5.28

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
73
A 100.0 mL sample of 0.10 mol L-1 Ca(OH)2 is titrated with 0.10 mol L-1 HBr. Determine the pH of the solution after the addition of 300.0 mL HBr.
A) 1.60
B) 1.30
C) 1.00
D) 12.40
E) 1.12
A) 1.60
B) 1.30
C) 1.00
D) 12.40
E) 1.12

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
74
A 100.0 mL sample of 0.20 mol L-1 HF is titrated with 0.10 mol L-1 KOH. Determine the pH of the solution after the addition of 300.0 mL of KOH. The Ka of HF is 3.5 × 10-4.
A) 12.40
B) 9.33
C) 5.06
D) 8.94
E) 12.00
A) 12.40
B) 9.33
C) 5.06
D) 8.94
E) 12.00

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following compounds' solubility will not be affected by a low pH in solution?
A) AgCl
B) Mg(OH)2
C) CaF2
D) CuS
E) BaCO3
A) AgCl
B) Mg(OH)2
C) CaF2
D) CuS
E) BaCO3

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
76
A 100.0 mL sample of 0.10 mol L-1 NH3 is titrated with 0.10 mol L-1 HNO3. Determine the pH of the solution before the addition of any HNO3. The Kb of NH3 is 1.8 × 10-5.
A) 4.74
B) 9.26
C) 11.13
D) 13.00
E) 12.55
A) 4.74
B) 9.26
C) 11.13
D) 13.00
E) 12.55

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
77
A 100.0 mL sample of 0.10 mol L-1 Ca(OH)2 is titrated with 0.10 mol L-1 HBr. Determine the pH of the solution after the addition of 200.0 mL HBr.
A) 2.62
B) 2.00
C) 1.48
D) 12.52
E) 7.00
A) 2.62
B) 2.00
C) 1.48
D) 12.52
E) 7.00

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
78
A 100.0 mL sample of 0.10 mol L-1 Ca(OH)2 is titrated with 0.10 mol L-1 HBr. Determine the pH of the solution before the addition of any HBr.
A) 12.86
B) 13.00
C) 13.30
D) 0.70
E) 1.00
A) 12.86
B) 13.00
C) 13.30
D) 0.70
E) 1.00

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
79
A 100.0 mL sample of 0.10 mol L-1 Ca(OH)2 is titrated with 0.10 mol L-1 HBr. Determine the pH of the solution after the addition of 100.0 mL HBr.
A) 2.00
B) 12.00
C) 1.30
D) 12.70
E) 7.00
A) 2.00
B) 12.00
C) 1.30
D) 12.70
E) 7.00

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following compounds will be more soluble in acidic solution than in pure water?
A) PbCl2
B) FeS
C) Ca(ClO4)2
D) CuI
E) AgBr
A) PbCl2
B) FeS
C) Ca(ClO4)2
D) CuI
E) AgBr

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck


