Exam 16: Aqueous Ionic Equilibrium
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
What is the pH of a buffer solution that is 0.295 mol L-1 in hypochlorous acid (HClO) and 0.373 mol L-1 in sodium hypochlorite (NaClO)? The 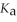 of hypochlorous acid is 3.8 ×
of hypochlorous acid is 3.8 × 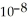 .
.
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
E
What is the molar solubility of AgCl in 0. 40 mol L-1 NH3? Ksp for AgCl is 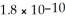 and Kf for Ag(NH3)2+ is
and Kf for Ag(NH3)2+ is 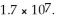
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
C
Determine the molar solubility of AgBr in a solution containing 0.150 mol L-1 NaBr. Ksp (AgBr) = 7.7 × 10-13.
Free
(Multiple Choice)
4.9/5  (24)
(24)
Correct Answer:
D
Calculate the percent ionization of nitrous acid in a solution that is 0.249 mol L-1 in nitrous acid. The acid dissociation constant of nitrous acid is 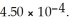
(Multiple Choice)
4.7/5  (27)
(27)
What is the pH of the resulting solution if 25.00 mL of 0.10 mol L-1 acetic acid is added to 10.00 mL of 0.10 mol L-1 NaOH? Assume that the volumes of the solutions are additive. Ka = 1.8 × 10-5 for CH3COOH 
(Multiple Choice)
4.7/5  (42)
(42)
What is the maximum ratio of conjugate base to acid in an effective buffer?
(Multiple Choice)
4.7/5  (27)
(27)
A solution containing CaCl2 is mixed with a solution of Li2C2O4 to form a solution that is 3.5 × 10-4 mol L-1 in calcium ion and 2.33 × 10-4 mol L-1 in oxalate ion. What will happen once these solutions are mixed? Ksp (CaC2O4) = 2.3 × 10-9.
(Multiple Choice)
4.7/5  (29)
(29)
What is the pH of a solution made by mixing 40.00 mL of 0.100 mol L-1 HCl with 25.00 mL of 0.100 mol L-1 KOH? Assume that the volumes of the solutions are additive.
(Multiple Choice)
4.8/5  (32)
(32)
What is the pH of a buffer system prepared by dissolving 10.70 grams of NH4Cl and 25.00 mL of 12 mol L-1 NH3 in enough water to make 1.000 L of solution? Kb = 1.80 × 10-5 for NH3.
(Multiple Choice)
4.9/5  (34)
(34)
Calculate the Ksp for silver carbonate if the solubility of Ag2CO3 in pure water is 3.5 × 10-2 g L-1.
(Multiple Choice)
4.8/5  (36)
(36)
When titrating a strong monoprotic acid with KOH at 25 °C, the ________.
(Multiple Choice)
4.8/5  (31)
(31)
A sample contains Ba3(PO4)2, CdS, AgCl, NH4Cl, and ZnS. Identify the precipitate formed after the addition of 6 mol L-1 HCl followed by the addition of H2S and 0.2 mol L-1 HCl.
(Multiple Choice)
4.8/5  (37)
(37)
A solution containing CaCl2 is mixed with a solution of Li2C2O4 to form a solution that is 2.1 × 10-5 mol L-1 in calcium ion and 4.75 × 10-5 mol L-1 in oxalate ion. What will happen once these solutions are mixed? Ksp (CaC2O4) = 2.3 × 10-9.
(Multiple Choice)
4.9/5  (35)
(35)
A 100.0 mL sample of 0.20 mol L-1 HF is titrated with 0.10 mol L-1 KOH. Determine the pH of the solution after the addition of 300.0 mL of KOH. The Ka of HF is 3.5 × 10-4.
(Multiple Choice)
4.9/5  (30)
(30)
Consider the Ksp values for two compounds: MZ, Ksp = 1.5 × 10-20 and MZ2, Ksp = 1.5 × 10-20. Why don't these compounds have the same molar solubility?
(Essay)
4.9/5  (39)
(39)
A solution of NaF is added dropwise to a solution that is 0.0188 mol L-1 in 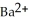 . When the concentration of
. When the concentration of 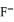 exceeds ________ mol L-1,
exceeds ________ mol L-1, 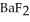 will precipitate. Neglect volume changes. For
will precipitate. Neglect volume changes. For 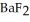 ,
, 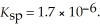
(Multiple Choice)
4.9/5  (43)
(43)
A sample contains Ba3(PO4)2, CdS, AgCl, NH4Cl, and ZnS. Identify the precipitate formed after the addition of 6 mol L-1 HCl, followed by the addition of H2S and 0.2 mol L-1 HCl, and subsequently the addition of OH- to a pH of 8.
(Multiple Choice)
4.8/5  (42)
(42)
Showing 1 - 20 of 173
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)