Deck 11: Liquids, Solids, and Intermolecular Forces
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/144
Play
Full screen (f)
Deck 11: Liquids, Solids, and Intermolecular Forces
1
Which pairs of substances would form homogeneous solutions when combined?
A) Methanol and water
B) Diethyl ether and water
C) CH2Cl2 and water
D) CCl4 and water
A) Methanol and water
B) Diethyl ether and water
C) CH2Cl2 and water
D) CCl4 and water
Methanol and water
2
Which one of the following has a definite shape and volume?
A) gas
B) liquid
C) solid
D) none of the above
A) gas
B) liquid
C) solid
D) none of the above
solid
3
Choose the compound that exhibits hydrogen bonding as its strongest intermolecular force.
A) SCl2
B) C2H6
C) CH3OH
D) CH2F2
E) None of the above compounds exhibit hydrogen bonding.
A) SCl2
B) C2H6
C) CH3OH
D) CH2F2
E) None of the above compounds exhibit hydrogen bonding.
CH3OH
4
Give the change in condition to go from a liquid to a gas.
A) increase heat or reduce pressure
B) increase heat or increase pressure
C) cool or reduce pressure
D) cool or increase pressure
E) none of the above
A) increase heat or reduce pressure
B) increase heat or increase pressure
C) cool or reduce pressure
D) cool or increase pressure
E) none of the above

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
5
Which pairs of substances would form homogeneous solutions when combined?
A) Hexane and water
B) Oil and water
C) KCl and water
D) CCl4 and water
A) Hexane and water
B) Oil and water
C) KCl and water
D) CCl4 and water

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
6
Place the following compounds in order of decreasing strength of intermolecular forces. HF O2 CO2
A) HF > CO2 > O2
B) HF > O2 > CO2
C) O2 > CO2 > HF
D) CO2 > HF > O2
E) CO2 > O2 > HF
A) HF > CO2 > O2
B) HF > O2 > CO2
C) O2 > CO2 > HF
D) CO2 > HF > O2
E) CO2 > O2 > HF

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
7
Which pairs of substances would form homogeneous solutions when combined?
A) CH2Cl2 and water
B) Diethyl ether and water
C) Glucose and water
D) CCl4 and water
A) CH2Cl2 and water
B) Diethyl ether and water
C) Glucose and water
D) CCl4 and water

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
8
Identify the characteristics of a liquid.
A) indefinite shape and volume
B) indefinite shape, but definite volume
C) definite shape and volume
D) definite shape and indefinite volume
A) indefinite shape and volume
B) indefinite shape, but definite volume
C) definite shape and volume
D) definite shape and indefinite volume

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
9
Place the following compounds in order of increasing strength of intermolecular forces. CO2 F2 NH2CH3
A) NH2CH3 < CO2 < F2
B) F2 < NH2CH3 < CO2
C) NH2CH3 < F2 < CO2
D) F2 < CO2 < NH2CH3
E) CO2 < NH2CH3 < F2
A) NH2CH3 < CO2 < F2
B) F2 < NH2CH3 < CO2
C) NH2CH3 < F2 < CO2
D) F2 < CO2 < NH2CH3
E) CO2 < NH2CH3 < F2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
10
What is the strongest type of intermolecular force present in H2?
A) ion-dipole
B) dipole-dipole
C) dispersion
D) hydrogen bonding
E) none of the above
A) ion-dipole
B) dipole-dipole
C) dispersion
D) hydrogen bonding
E) none of the above

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
11
Choose the molecule or compound that exhibits dispersion forces as its strongest intermolecular force.
A) Cl2
B) NH3
C) HF
D) NaCl
A) Cl2
B) NH3
C) HF
D) NaCl

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
12
The forces between polar molecules is known as ________.
A) hydrogen bonding
B) ion-dipole forces
C) dipole-dipole forces
D) dispersion forces
E) ionic forces
A) hydrogen bonding
B) ion-dipole forces
C) dipole-dipole forces
D) dispersion forces
E) ionic forces

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following statements is TRUE?
A) Intermolecular forces are generally stronger than bonding forces.
B) The potential energy of molecules decrease as they get closer to one another.
C) Energy is given off when the attraction between two molecules is broken.
D) Increasing the pressure on a solid usually causes it to become a liquid.
E) None of the above is true.
A) Intermolecular forces are generally stronger than bonding forces.
B) The potential energy of molecules decrease as they get closer to one another.
C) Energy is given off when the attraction between two molecules is broken.
D) Increasing the pressure on a solid usually causes it to become a liquid.
E) None of the above is true.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
14
What type of intermolecular force causes the dissolution of NaCl in water?
A) hydrogen bonding
B) dipole-dipole forces
C) ion-dipole force
D) dispersion forces
E) none of the above
A) hydrogen bonding
B) dipole-dipole forces
C) ion-dipole force
D) dispersion forces
E) none of the above

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
15
Choose the molecule or compound that exhibits dipole-dipole forces as its strongest intermolecular force.
A) H2
B) SO2
C) NH3
D) CF4
E) BCl3
A) H2
B) SO2
C) NH3
D) CF4
E) BCl3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
16
What is the strongest type of intermolecular force present in CHF3?
A) ion-dipole
B) dispersion
C) hydrogen bonding
D) dipole-dipole
E) none of the above
A) ion-dipole
B) dispersion
C) hydrogen bonding
D) dipole-dipole
E) none of the above

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
17
What is the strongest type of intermolecular force present in NH2CH3?
A) dispersion
B) dipole-dipole
C) hydrogen bonding
D) ion-dipole
E) none of the above
A) dispersion
B) dipole-dipole
C) hydrogen bonding
D) ion-dipole
E) none of the above

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following has a low density?
A) gas
B) liquid
C) solid
D) none of the above
A) gas
B) liquid
C) solid
D) none of the above

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
19
Place the following compounds in order of increasing strength of intermolecular forces. CH4 CH3CH2CH3 CH3CH3
A) CH3CH2CH3 < CH4 < CH3CH3
B) CH3CH2CH3 < CH3CH3 < CH4
C) CH3CH3 < CH4 < CH3CH2CH3
D) CH4 < CH3CH2CH3 < CH3CH3
E) CH4 < CH3CH3 < CH3CH2CH3
A) CH3CH2CH3 < CH4 < CH3CH3
B) CH3CH2CH3 < CH3CH3 < CH4
C) CH3CH3 < CH4 < CH3CH2CH3
D) CH4 < CH3CH2CH3 < CH3CH3
E) CH4 < CH3CH3 < CH3CH2CH3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
20
What type of intermolecular force causes the dissolution of CH3CH2OH in water?
A) hydrogen bonding
B) dipole-dipole forces
C) ion-dipole forces
D) dispersion forces
E) none of the above
A) hydrogen bonding
B) dipole-dipole forces
C) ion-dipole forces
D) dispersion forces
E) none of the above

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
21
Choose the substance with the lowest surface tension in the liquid phase.
A) CH3SH
B) CH3CH2CH2CH3
C) C6H6
D) H2O
E) (CH3)2CO
A) CH3SH
B) CH3CH2CH2CH3
C) C6H6
D) H2O
E) (CH3)2CO

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
22
Choose the pair of substances that are most likely to form a homogeneous solution.
A) CCl4 and SCl2
B) NF3 and SO2
C) CO and C6H6
D) NH2CH3 and CH4
E) None of the pairs above will form a homogeneous solution.
A) CCl4 and SCl2
B) NF3 and SO2
C) CO and C6H6
D) NH2CH3 and CH4
E) None of the pairs above will form a homogeneous solution.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
23
Define boiling.
A) A liquid becomes a gas.
B) A gas becomes a liquid.
C) A gas becomes a solid.
D) A solid becomes a gas.
E) A solid becomes a liquid.
A) A liquid becomes a gas.
B) A gas becomes a liquid.
C) A gas becomes a solid.
D) A solid becomes a gas.
E) A solid becomes a liquid.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
24
Identify the compound that does not have dipole-dipole forces as its strongest force.
A) CH2Cl2
B) CH3OCH3
C) CH3Br
D) HCCl3
E) CO2
A) CH2Cl2
B) CH3OCH3
C) CH3Br
D) HCCl3
E) CO2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
25
Choose the substance with the highest surface tension in the liquid phase.
A) HOCH2CH2OH
B) CH2F2
C) CH3CH2F
D) CH3CH2OH
E) CH3CH2CH3
A) HOCH2CH2OH
B) CH2F2
C) CH3CH2F
D) CH3CH2OH
E) CH3CH2CH3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
26
Choose the pair of substances that are most likely to form a homogeneous solution.
A) NaCl and Hg
B) LiF and C6H14
C) C3H8 and C2H5OH
D) Br2 and PF3
E) NH3 and CH3OH
A) NaCl and Hg
B) LiF and C6H14
C) C3H8 and C2H5OH
D) Br2 and PF3
E) NH3 and CH3OH

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
27
Choose the substance with the highest viscosity in the liquid phase.
A) (CH3CH2)2CO
B) C2H4Cl2
C) HOCH2CH2CH2CH2OH
D) CF4
E) C6H14
A) (CH3CH2)2CO
B) C2H4Cl2
C) HOCH2CH2CH2CH2OH
D) CF4
E) C6H14

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
28
Which substance below has the strongest intermolecular forces?
A) A2X, ΔvapH = 39.6 kJ mol-1
B) BY2, ΔvapH = 26.7 kJ mol-1
C) C3X2, ΔvapH = 36.4 kJ mol-1
D) DX2, ΔvapH = 23.3 kJ mol-1
E) EY3, ΔvapH = 21.5 kJ mol-1
A) A2X, ΔvapH = 39.6 kJ mol-1
B) BY2, ΔvapH = 26.7 kJ mol-1
C) C3X2, ΔvapH = 36.4 kJ mol-1
D) DX2, ΔvapH = 23.3 kJ mol-1
E) EY3, ΔvapH = 21.5 kJ mol-1

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
29
Give the term for the temperature at which the gas and liquid phases form a supercritical fluid.
A) absolute temperature
B) definite temperature
C) fluid temperature
D) critical temperature
E) solid temperature
A) absolute temperature
B) definite temperature
C) fluid temperature
D) critical temperature
E) solid temperature

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
30
Identify the substance with the highest viscosity in the liquid phase.
A) gasoline
B) water
C) corn syrup
D) motor oil
E) tea
A) gasoline
B) water
C) corn syrup
D) motor oil
E) tea

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
31
Choose the substance with the lowest viscosity in the liquid phase.
A) Cl3CCCl3
B) Cl2CHCH2Cl
C) Cl2CHCHCl2
D) ClCH2CH2Cl
E) Cl3CCHCl2
A) Cl3CCCl3
B) Cl2CHCH2Cl
C) Cl2CHCHCl2
D) ClCH2CH2Cl
E) Cl3CCHCl2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
32
Choose the substance with the highest viscosity in the liquid phase.
A) SbCl3
B) AsCl5
C) ICl2
D) BeCl2
E) OCl2
A) SbCl3
B) AsCl5
C) ICl2
D) BeCl2
E) OCl2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following statements is FALSE?
A) The rate of vaporization increases with increasing surface area.
B) The rate of vaporization increases with decreasing strength of intermolecular forces.
C) The rate of vaporization increases with increasing temperature.
D) Molecules with hydrogen bonding are more volatile than compounds with dipole-dipole forces.
A) The rate of vaporization increases with increasing surface area.
B) The rate of vaporization increases with decreasing strength of intermolecular forces.
C) The rate of vaporization increases with increasing temperature.
D) Molecules with hydrogen bonding are more volatile than compounds with dipole-dipole forces.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
34
The energy required to increase the surface area of a liquid by a unit amount is called ________.
A) viscosity
B) surface tension
C) dipole-dipole force
D) hydrogen bonding
E) capillary action
A) viscosity
B) surface tension
C) dipole-dipole force
D) hydrogen bonding
E) capillary action

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
35
Place the following compounds in order of decreasing strength of intermolecular forces. I. CH3CH2CH2CH2CH2CH3 II. (CH3)3CCH3 III. (CH3)3CCH2CH3
A) III > II > I
B) I > III > II
C) I > II > III
D) II > III > I
E) III > I > II
A) III > II > I
B) I > III > II
C) I > II > III
D) II > III > I
E) III > I > II

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
36
Identify the term used to describe the ability of a liquid to flow against gravity up a narrow tube.
A) capillary action
B) viscosity
C) surface tension
D) density
A) capillary action
B) viscosity
C) surface tension
D) density

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
37
Choose the pair of substances that are most likely to form a homogeneous solution.
A) C6H14 and C10H20
B) LiBr and C5H12
C) N2O4 and NH4Cl
D) C6H14 and H2O
E) None of the pairs above will form a homogeneous solution.
A) C6H14 and C10H20
B) LiBr and C5H12
C) N2O4 and NH4Cl
D) C6H14 and H2O
E) None of the pairs above will form a homogeneous solution.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements is TRUE?
A) Vapour pressure increases with temperature.
B) Hydrogen bonds are stronger than covalent bonds.
C) Intermolecular forces hold the atoms in molecules together.
D) Dispersion forces are generally stronger than dipole-dipole forces.
A) Vapour pressure increases with temperature.
B) Hydrogen bonds are stronger than covalent bonds.
C) Intermolecular forces hold the atoms in molecules together.
D) Dispersion forces are generally stronger than dipole-dipole forces.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
39
Choose the substance with the highest vapour pressure at a given temperature.
A) SiS2
B) RbCl
C) CH3SCH3
D) BF3
E) SbH3
A) SiS2
B) RbCl
C) CH3SCH3
D) BF3
E) SbH3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
40
Identify the compound that does not have hydrogen bonding.
A) (CH3)3N
B) H2O
C) CH3OH
D) HF
E) CH3NH2
A) (CH3)3N
B) H2O
C) CH3OH
D) HF
E) CH3NH2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
41
Place the following substances in order of decreasing vapour pressure at a given temperature. PF5 BrF3 CF4
A) BrF3 > PF5 > CF4
B) BrF3 > CF4 > PF5
C) PF5 > BrF3 > CF4
D) CF4 > BrF3 > PF5
E) CF4 > PF5 > BrF3
A) BrF3 > PF5 > CF4
B) BrF3 > CF4 > PF5
C) PF5 > BrF3 > CF4
D) CF4 > BrF3 > PF5
E) CF4 > PF5 > BrF3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
42
Place the following substances in order of increasing boiling point. CH3CH2OH Ar CH3OCH3
A) Ar < CH3OCH3 < CH3CH2OH
B) CH3CH2OH < Ar < CH3OCH3
C) CH3CH2OH < CH3OCH3 < Ar
D) CH3OCH3 < Ar < CH3CH2OH
E) Ar < CH3CH2OH < CH3OCH3
A) Ar < CH3OCH3 < CH3CH2OH
B) CH3CH2OH < Ar < CH3OCH3
C) CH3CH2OH < CH3OCH3 < Ar
D) CH3OCH3 < Ar < CH3CH2OH
E) Ar < CH3CH2OH < CH3OCH3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
43
Choose the substance with the highest boiling point.
A) Acetone
B) Ethanol
C) Methanol
D) Propanol
E) Butanol
A) Acetone
B) Ethanol
C) Methanol
D) Propanol
E) Butanol

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
44
Choose the substance with the lowest boiling point.
A) H2S
B) NBr3
C) F2
D) CF2H2
E) H2O2
A) H2S
B) NBr3
C) F2
D) CF2H2
E) H2O2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
45
Choose the substance with the lowest boiling point.
A) Acetone
B) Ethanol
C) Methanol
D) Propanol
E) Butanol
A) Acetone
B) Ethanol
C) Methanol
D) Propanol
E) Butanol

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
46
Choose the substance with the highest boiling point.
A) CH4
B) KI
C) CS2
D) HF
E) I2
A) CH4
B) KI
C) CS2
D) HF
E) I2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
47
Place the following substances in order of decreasing vapour pressure at a given temperature. BeF2 CH3OH OF2
A) CH3OH > OF2 > BeF2
B) BeF2 > OF2 > CH3OH
C) OF2 > CH3OH > BeF2
D) OF2 > BeF2 > CH3OH
E) BeF2 > CH3OH > OF2
A) CH3OH > OF2 > BeF2
B) BeF2 > OF2 > CH3OH
C) OF2 > CH3OH > BeF2
D) OF2 > BeF2 > CH3OH
E) BeF2 > CH3OH > OF2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following substances would you predict to have the highest ΔvapH?
A) CH3Cl
B) HCl
C) HOCH2CH2OH
D) CH3CH2OH
E) CH3CH2CH2CH3
A) CH3Cl
B) HCl
C) HOCH2CH2OH
D) CH3CH2OH
E) CH3CH2CH2CH3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
49
Place the following substances in order of decreasing boiling point. N2 O2 H2
A) O2 > H2 > N2
B) N2 > H2 > O2
C) N2 > O2 > H2
D) O2 > N2 > H2
E) H2 > N2 > O2
A) O2 > H2 > N2
B) N2 > H2 > O2
C) N2 > O2 > H2
D) O2 > N2 > H2
E) H2 > N2 > O2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
50
Choose the substance with the lowest boiling point.
A) Diethyl ether
B) Ethanol
C) Methanol
D) Propanol
E) Butanol
A) Diethyl ether
B) Ethanol
C) Methanol
D) Propanol
E) Butanol

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
51
Place the following substances in order of decreasing boiling point. H2O N2 CO
A) CO > H2O > N2
B) N2 > CO > H2O
C) H2O > CO > N2
D) CO > N2 > H2O
E) N2 > H2O > CO
A) CO > H2O > N2
B) N2 > CO > H2O
C) H2O > CO > N2
D) CO > N2 > H2O
E) N2 > H2O > CO

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
52
How much energy is required to vaporize 158 g of butane (C4H10) at its boiling point if its ΔvapH is 24.3 kJ mol-1?
A) 15.1 kJ
B) 66.1 kJ
C) 89.4 kJ
D) 11.2 kJ
E) 38.4 kJ
A) 15.1 kJ
B) 66.1 kJ
C) 89.4 kJ
D) 11.2 kJ
E) 38.4 kJ

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
53
Place the following substances in order of increasing vapour pressure at a given temperature. NF3 NH3 BCl3
A) NH3 < NF3 < BCl3
B) NF3 < NH3 < BCl3
C) BCl3 < NF3 < NH3
D) NH3 < BCl3 < NF3
E) BCl3 < NH3 < NF3
A) NH3 < NF3 < BCl3
B) NF3 < NH3 < BCl3
C) BCl3 < NF3 < NH3
D) NH3 < BCl3 < NF3
E) BCl3 < NH3 < NF3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
54
Choose the substance with the highest boiling point.
A) CH4
B) CH3CH3
C) CH3CH2Cl
D) CH3CH2CH2OH
E) H2
A) CH4
B) CH3CH3
C) CH3CH2Cl
D) CH3CH2CH2OH
E) H2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
55
How much energy is required to vaporize 98.6 g of ethanol (C2H5OH) at its boiling point if its ΔvapH is 40.5 kJ mol-1?
A) 86.7 kJ
B) 11.5 kJ
C) 18.9 kJ
D) 52.8 kJ
E) 39.9 kJ
A) 86.7 kJ
B) 11.5 kJ
C) 18.9 kJ
D) 52.8 kJ
E) 39.9 kJ

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
56
Place the following substances in order of increasing vapour pressure at a given temperature. SF6 SiH4 SF4
A) SF6 < SiH4 < SF4
B) SiH4 < SF4 < SF6
C) SF6 < SF4 < SiH4
D) SF4 < SF6 < SiH4
E) SiH4 < SF6 < SF4
A) SF6 < SiH4 < SF4
B) SiH4 < SF4 < SF6
C) SF6 < SF4 < SiH4
D) SF4 < SF6 < SiH4
E) SiH4 < SF6 < SF4

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
57
Choose the substance with the lowest vapour pressure at a given temperature.
A) CO2
B) BeCl2
C) BF3
D) He
E) PF5
A) CO2
B) BeCl2
C) BF3
D) He
E) PF5

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
58
How much energy is required to vaporize 48.7 g of dichloromethane (CH2Cl2) at its boiling point if its ΔvapH is 31.6 kJ mol-1?
A) 31.2 kJ
B) 6.49 kJ
C) 55.1 kJ
D) 15.4 kJ
E) 18.1 kJ
A) 31.2 kJ
B) 6.49 kJ
C) 55.1 kJ
D) 15.4 kJ
E) 18.1 kJ

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following substances would you predict to have the highest ΔvapH?
A) Xe
B) C6H6
C) SiF4
D) Br2
E) N2
A) Xe
B) C6H6
C) SiF4
D) Br2
E) N2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
60
Place the following substances in order of increasing boiling point. Ne Cl2 O2
A) Ne < Cl2 < O2
B) Cl2 < O2 < Ne
C) O2 < Cl2 < Ne
D) Cl2 < Ne < O2
E) Ne < O2 < Cl2
A) Ne < Cl2 < O2
B) Cl2 < O2 < Ne
C) O2 < Cl2 < Ne
D) Cl2 < Ne < O2
E) Ne < O2 < Cl2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
61
Place the following substances in order of decreasing boiling point. He Ar H2
A) He > H2 > Ar
B) H2 > He > Ar
C) He > Ar > H2
D) Ar > He > H2
E) H2 > Ar > He
A) He > H2 > Ar
B) H2 > He > Ar
C) He > Ar > H2
D) Ar > He > H2
E) H2 > Ar > He

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
62
How much energy is required to heat 36.0 g H2O from a liquid at 65 °C to a gas at 115 °C? The following physical data may be useful: ΔvapH = 40.7 kJ mol-1
Cliq = 4.18 J g-1 °C-1
Cgas = 2.01 J g-1 °C-1
Csol = 2.09 J g-1 °C-1
Tmelting = 0 ∘C
Tboiling = 100 ∘C
A) 63.5 kJ
B) 87.7 kJ
C) 10.9 kJ
D) 52.7 kJ
E) 91.7 kJ
Cliq = 4.18 J g-1 °C-1
Cgas = 2.01 J g-1 °C-1
Csol = 2.09 J g-1 °C-1
Tmelting = 0 ∘C
Tboiling = 100 ∘C
A) 63.5 kJ
B) 87.7 kJ
C) 10.9 kJ
D) 52.7 kJ
E) 91.7 kJ

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
63
Determine the vapour pressure (in mbar) of a substance at 36 °C whose normal boiling point is 84 °C and has a ΔvapH of 22.1 kJ mol-1.
A) 319 mbar
B) 31.8 mbar
C) 41.8 mbar
D) 147 mbar
E) 98 mbar
A) 319 mbar
B) 31.8 mbar
C) 41.8 mbar
D) 147 mbar
E) 98 mbar

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
64
Determine the normal boiling point of a substance whose vapour pressure is 73.46 mbar at 35 °C and has a ΔvapH of 32.1 kJ mol-1.
A) 255 K
B) 368 K
C) 412 K
D) 390. K
E) 466 K
A) 255 K
B) 368 K
C) 412 K
D) 390. K
E) 466 K

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
65
The normal boiling point of water is ________.
A) 0 °C
B) 32 °C
C) 212 °C
D) 100 °C
E) 273 °C
A) 0 °C
B) 32 °C
C) 212 °C
D) 100 °C
E) 273 °C

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
66
The melting point of water is ________.
A) 0 °C
B) 32 °C
C) 212 °C
D) 100 °C
E) 273 °C
A) 0 °C
B) 32 °C
C) 212 °C
D) 100 °C
E) 273 °C

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
67
The freezing point of water is ________.
A) 0 °C
B) 32 °C
C) 212 °C
D) 100 °C
E) 273 °C
A) 0 °C
B) 32 °C
C) 212 °C
D) 100 °C
E) 273 °C

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
68
Define freezing.
A) the phase transition from solid to gas
B) the phase transition from gas to solid
C) the phase transition from gas to liquid
D) the phase transition from liquid to gas
E) the phase transition from liquid to solid
A) the phase transition from solid to gas
B) the phase transition from gas to solid
C) the phase transition from gas to liquid
D) the phase transition from liquid to gas
E) the phase transition from liquid to solid

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
69
Determine ΔvapH for a compound that has a measured vapour pressure of 32.40 mbar at 273 K and 180.0 mbar at 325 K.
A) 41 kJ mol-1
B) 79 kJ mol-1
C) 24 kJ mol-1
D) 13 kJ mol-1
E) 34 kJ mol-1
A) 41 kJ mol-1
B) 79 kJ mol-1
C) 24 kJ mol-1
D) 13 kJ mol-1
E) 34 kJ mol-1

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
70
Determine the vapour pressure (in mbar) of a substance at 29 °C, whose normal boiling point is 76 °C and has a ΔvapH of 38.7 kJ mol-1.
A) 80 mbar
B) 13 mbar
C) 21 mbar
D) 48 mbar
E) 128 mbar
A) 80 mbar
B) 13 mbar
C) 21 mbar
D) 48 mbar
E) 128 mbar

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
71
How much energy must be removed from a 125 g sample of benzene (molar mass = 78.11 g mol-1) at 425.0 K to liquify the sample and lower the temperature to 335.0 K? The following physical data may be useful: ΔvapH = 33.9 kJ mol-1
ΔfusH = 9.8 kJ mol-1
Cliq = 1.73 J g-1 °C-1
Cgas = 1.06 J g-1 °C-1
Csol = 1.51 J g-1 °C-1
Tmelting = 279.0 K
Tboiling = 353.0 K
A) 38.9 kJ
B) 95.4 kJ
C) 67.7 kJ
D) 54.3 kJ
E) 74.4 kJ
ΔfusH = 9.8 kJ mol-1
Cliq = 1.73 J g-1 °C-1
Cgas = 1.06 J g-1 °C-1
Csol = 1.51 J g-1 °C-1
Tmelting = 279.0 K
Tboiling = 353.0 K
A) 38.9 kJ
B) 95.4 kJ
C) 67.7 kJ
D) 54.3 kJ
E) 74.4 kJ

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
72
How much energy must be removed from a 94.4 g sample of benzene (molar mass = 78.11 g mol-1) at 322.0 K to solidify the sample and lower the temperature to 205.0 K? The following physical data may be useful: ΔvapH = 33.9 kJ mol-1
ΔfusH = 9.8 kJ mol-1
Cliq = 1.73 J g-1 °C-1
Cgas = 1.06 J g-1 °C-1
Csol = 1.51 J g-1 °C-1
Tmelting = 279.0 K
Tboiling = 353.0 K
A) 17.6 kJ
B) 11.8 kJ
C) 70.2 kJ
D) 10.5 kJ
E) 29.4 kJ
ΔfusH = 9.8 kJ mol-1
Cliq = 1.73 J g-1 °C-1
Cgas = 1.06 J g-1 °C-1
Csol = 1.51 J g-1 °C-1
Tmelting = 279.0 K
Tboiling = 353.0 K
A) 17.6 kJ
B) 11.8 kJ
C) 70.2 kJ
D) 10.5 kJ
E) 29.4 kJ

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
73
Define sublimation.
A) the phase transition from solid to gas
B) the phase transition from gas to solid
C) the phase transition from gas to liquid
D) the phase transition from liquid to gas
E) the phase transition from liquid to solid
A) the phase transition from solid to gas
B) the phase transition from gas to solid
C) the phase transition from gas to liquid
D) the phase transition from liquid to gas
E) the phase transition from liquid to solid

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the phase diagram shown. Choose the statement below that is TRUE. 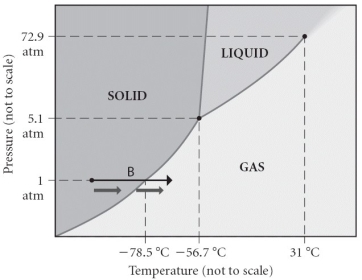
A) The triple point of this substance occurs at a temperature of 31 °C.
B) At 10 bar of pressure, there is no temperature where the liquid phase of this substance would exist.
C) The solid phase of this substance is higher in density than the liquid phase.
D) The line separating the solid and liquid phases represents the ΔvapH.

A) The triple point of this substance occurs at a temperature of 31 °C.
B) At 10 bar of pressure, there is no temperature where the liquid phase of this substance would exist.
C) The solid phase of this substance is higher in density than the liquid phase.
D) The line separating the solid and liquid phases represents the ΔvapH.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
75
Assign the appropriate labels to the phase diagram shown below. 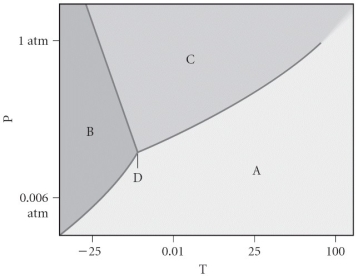
A) A = liquid, B = solid, C = gas, D = critical point
B) A = gas, B = solid, C = liquid, D = triple point
C) A = gas, B = liquid, C = solid, D = critical point
D) A = solid, B = gas, C = liquid, D = supercritical fluid
E) A = liquid, B = gas, C = solid, D = triple point

A) A = liquid, B = solid, C = gas, D = critical point
B) A = gas, B = solid, C = liquid, D = triple point
C) A = gas, B = liquid, C = solid, D = critical point
D) A = solid, B = gas, C = liquid, D = supercritical fluid
E) A = liquid, B = gas, C = solid, D = triple point

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
76
Identify triple point.
A) the temperature, pressure, and density for a gas
B) the temperature at which the boiling point equals the melting point
C) the temperature and pressure where liquid, solid, and gas are equally stable and are in equilibrium
D) the temperature that is unique for a substance
E) the temperature at which the solid and liquid coexist
A) the temperature, pressure, and density for a gas
B) the temperature at which the boiling point equals the melting point
C) the temperature and pressure where liquid, solid, and gas are equally stable and are in equilibrium
D) the temperature that is unique for a substance
E) the temperature at which the solid and liquid coexist

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
77
At atmospheric pressure, dry ice ________.
A) freezes
B) deposits
C) sublimes
D) melts
E) boils
A) freezes
B) deposits
C) sublimes
D) melts
E) boils

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
78
Define deposition.
A) A liquid becomes a gas.
B) A gas becomes a liquid.
C) A gas becomes a solid.
D) A solid becomes a gas.
E) A solid becomes a liquid.
A) A liquid becomes a gas.
B) A gas becomes a liquid.
C) A gas becomes a solid.
D) A solid becomes a gas.
E) A solid becomes a liquid.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
79
Consider the phase diagram below. If the dashed line at 1 atm of pressure is followed from 100 to 500 °C, what phase changes will occur (in order of increasing temperature)? 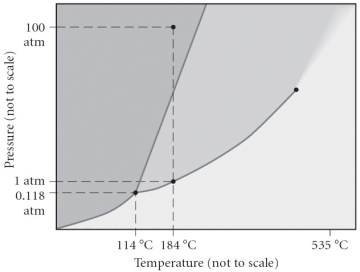
A) condensation followed by vaporization
B) sublimation followed by deposition
C) vaporization followed by deposition
D) fusion followed by vaporization
E) No phase change will occur under the conditions specified.

A) condensation followed by vaporization
B) sublimation followed by deposition
C) vaporization followed by deposition
D) fusion followed by vaporization
E) No phase change will occur under the conditions specified.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
80
How much energy is required to heat 87.1 g acetone (molar mass = 58.08 g mol-1) from a solid at -154.0 °C to a liquid at -42. 0°C? The following physical data may be useful: ΔfusH = 7.27 kJ mol-1
Cliq = 2.16 J g-1 °C-1
Cgas = 1.29 J g-1 °C-1
Csol = 1.65 J g-1 °C-1
Tmelting = -95.0 °C
A) 8.48 kJ
B) 18.5 kJ
C) 32.2 kJ
D) 29.4 kJ
E) 9.97 kJ
Cliq = 2.16 J g-1 °C-1
Cgas = 1.29 J g-1 °C-1
Csol = 1.65 J g-1 °C-1
Tmelting = -95.0 °C
A) 8.48 kJ
B) 18.5 kJ
C) 32.2 kJ
D) 29.4 kJ
E) 9.97 kJ

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck


