Exam 11: Liquids, Solids, and Intermolecular Forces
Exam 1: Units of Measurement for Physical and Chemical Change192 Questions
Exam 2: Atoms and Elements174 Questions
Exam 3: Molecules, Compounds, and Nomenclature187 Questions
Exam 4: Chemical Reactions and Stoichiometry261 Questions
Exam 5: Gases163 Questions
Exam 6: Thermochemistry161 Questions
Exam 7: The Quantum-Mechanical Model of the Atom170 Questions
Exam 8: Periodic Properties of the Elements144 Questions
Exam 9: Chemical Bonding I: Lewis Theory155 Questions
Exam 10: Chemical Bonding Ii: Molecular Shapes, Valence Bond Theory, and Molecular Orbital Theory180 Questions
Exam 11: Liquids, Solids, and Intermolecular Forces144 Questions
Exam 12: Solutions167 Questions
Exam 13: Chemical Kinetics170 Questions
Exam 14: Chemical Equilibrium150 Questions
Exam 15: Acids and Bases156 Questions
Exam 16: Aqueous Ionic Equilibrium173 Questions
Exam 17: Gibbs Energy and Thermodynamics134 Questions
Exam 18: Electrochemistry122 Questions
Exam 19: Radioactivity and Nuclear Chemistry116 Questions
Exam 20: Organic Chemistry I: Structures109 Questions
Exam 21: Organic Chemistry Ii: Reactions102 Questions
Exam 22: Biochemistry55 Questions
Exam 23: Chemistry of the Nonmetals50 Questions
Exam 24: Metals and Metallurgy49 Questions
Exam 25: Transition Metals and Coordination Compounds55 Questions
Select questions type
Why is the ΔvapH higher than ΔfusH for a given compound?
Free
(Essay)
4.9/5  (29)
(29)
Correct Answer:
Vaporizing a substance requires the complete "breaking" of all intermolecular attractions, whereas the melting of a substance only requires the breaking of a portion of the intermolecular attractions.
Determine the vapour pressure (in mbar) of a substance at 36 °C whose normal boiling point is 84 °C and has a ΔvapH of 22.1 kJ mol-1.
Free
(Multiple Choice)
4.8/5  (32)
(32)
Correct Answer:
A
The enthalpy change for converting 1.00 mol of ice at -60.0°C to water at 80.0°C is  The specific heats of ice, water, and steam are
The specific heats of ice, water, and steam are 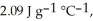
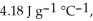 and
and 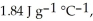 respectively. For
respectively. For 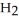 O,
O, 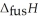 = 6.01 kJ mol-1, and
= 6.01 kJ mol-1, and 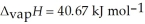 .
.
(Multiple Choice)
4.8/5  (38)
(38)
Describe the difference between the conduction band and the valence band.
(Essay)
4.8/5  (35)
(35)
Which of the following substances would you predict to have the highest ΔvapH?
(Multiple Choice)
4.7/5  (39)
(39)
Choose the compound that exhibits hydrogen bonding as its strongest intermolecular force.
(Multiple Choice)
4.9/5  (32)
(32)
The enthalpy change for converting 10.0 g of ice at -25.0 °C to water at 80.0 °C is ________ kJ. The specific heats of ice, water, and steam are 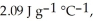
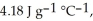 and
and 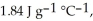 respectively. For
respectively. For 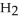 O,
O, 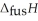 = 6.01 kJ mol-1, and
= 6.01 kJ mol-1, and 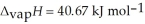 .
.
(Multiple Choice)
4.9/5  (44)
(44)
Identify the term used to describe the ability of a liquid to flow against gravity up a narrow tube.
(Multiple Choice)
4.9/5  (37)
(37)
Vanadium crystallizes in a body-centred cubic structure and has an atomic radius of 131 pm. Determine the density of vanadium if the edge length of a bcc structure is 4r/ 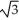 .
.
(Multiple Choice)
5.0/5  (38)
(38)
What type of intermolecular force causes the dissolution of CH3CH2OH in water?
(Multiple Choice)
4.8/5  (34)
(34)
Give the coordination number for a body-centred cubic cell.
(Multiple Choice)
4.8/5  (31)
(31)
Based on the figure above, the boiling point of diethyl ether under an external pressure of 1.32 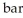 is ________ °C.
is ________ °C.
(Multiple Choice)
4.8/5  (28)
(28)
Choose the pair of substances that are most likely to form a homogeneous solution.
(Multiple Choice)
4.7/5  (30)
(30)
Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) at 35.0 °C to gaseous CCl4 at 76.8 °C (the normal boiling point for CCl4). The specific heat of CCl4(l) is 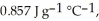 its heat of fusion is
its heat of fusion is 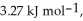 and its heat of vaporization is
and its heat of vaporization is 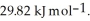
(Multiple Choice)
4.9/5  (52)
(52)
How much energy is required to vaporize 98.6 g of ethanol (C2H5OH) at its boiling point if its ΔvapH is 40.5 kJ mol-1?
(Multiple Choice)
4.8/5  (34)
(34)
Showing 1 - 20 of 144
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)