Deck 2: Chemical Components of Cells
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Premises:
Responses:
False
True
False
True
False
True
Question
Question
Question
Question
Match between columns
Premises:
Responses:
True
False
True
False
True
False
Question
Question
Question
Match between columns
Premises:
Responses:
True
False
True
False
True
False
Question
Question
Question
Match between columns
Premises:
Responses:
True
False
True
False
True
False
Question
Question
Match between columns
Premises:
Responses:
False
True
False
True
False
True
False
True
Question
Match between columns
Premises:
Responses:
False
True
False
True
False
True
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Premises:
Responses:
False
True
False
True
False
True
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/74
Play
Full screen (f)
Deck 2: Chemical Components of Cells
1
Select the answer that BEST completes the following statement: Chemical reactions in living systems occur in an __________ environment, within a narrow range of temperatures.
A)optimal
B)organic
C)extracellular
D)aqueous
A)optimal
B)organic
C)extracellular
D)aqueous
D
2
If the isotope 32S has 16 protons and 16 neutrons, how many protons, neutrons, and electrons will the isotope 35S have, respectively?
A)16; 20; 15
B)16; 19; 15
C)16; 19; 16
D)16; 19; 17
A)16; 20; 15
B)16; 19; 15
C)16; 19; 16
D)16; 19; 17
C
3
Avogadro's number was established as the total number of units (atoms or molecules) in a mole, and a mole of any substance is X grams of it, where X is equal to the substance's molecular weight.A standard unit, the mole, allows scientists to calculate concentrations of materials dissolved in solutions.
Example: Sulfur has a molecular weight of 32.Therefore, 32 g of sulfur = 1 mole of sulfur = 6 × 1023 sulfur atoms.
How many moles and atoms, respectively, are there in 120 grams of sulfur?
A)3.75; 6 × 1023
B)32; 6 × 1023
C)1.75; 1.05 × 1024
D)3.75; 2.25 × 1024
Example: Sulfur has a molecular weight of 32.Therefore, 32 g of sulfur = 1 mole of sulfur = 6 × 1023 sulfur atoms.
How many moles and atoms, respectively, are there in 120 grams of sulfur?
A)3.75; 6 × 1023
B)32; 6 × 1023
C)1.75; 1.05 × 1024
D)3.75; 2.25 × 1024
D
4
There are 90 naturally occurring elements on the earth, __________ of which compose 96% of the mass of living organisms.
A)4
B)6
C)7
D)8
A)4
B)6
C)7
D)8

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
5
Carbon 14 is an unstable isotope of carbon that decays very slowly.Compared to the common, stable carbon 12 isotope, carbon 14 has two additional
A)electrons.
B)neutrons.
C)protons.
D)ions.
A)electrons.
B)neutrons.
C)protons.
D)ions.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
6
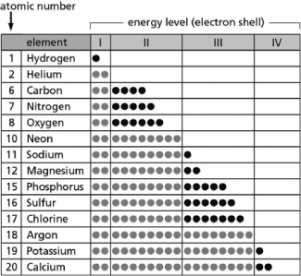 Table 2-14
Table 2-14-Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.On the basis of the information in the chart and what you know about atomic structure, which elements will form ions with a net charge of +2 in solution?
A)carbon; sulfur
B)helium; neon
C)sodium; potassium
D)magnesium; calcium

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
7
Which subatomic particles contribute to the atomic number for any given element?
A)protons
B)protons and neutrons
C)neutrons
D)protons and electrons
A)protons
B)protons and neutrons
C)neutrons
D)protons and electrons

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
8
Which subatomic particles can vary between isotopes of the same element, without changing the observed chemical properties?
A)electrons
B)protons and neutrons
C)neutrons
D)neutrons and electrons
A)electrons
B)protons and neutrons
C)neutrons
D)neutrons and electrons

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
9
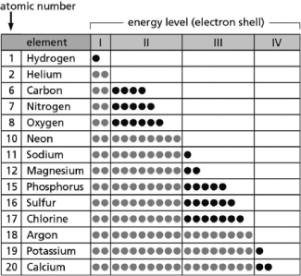 Table 2-14
Table 2-14-Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.On the basis of the information in the chart and what you know about atomic structure, which elements form stable but reactive diatomic gases?
A)nitrogen; oxygen
B)helium; neon
C)sodium; potassium
D)magnesium; calcium

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
10
An ionic bond between two atoms is formed as a result of the
A)sharing of electrons.
B)loss of electrons from both atoms.
C)loss of a proton from one atom.
D)transfer of electrons from one atom to the other.
A)sharing of electrons.
B)loss of electrons from both atoms.
C)loss of a proton from one atom.
D)transfer of electrons from one atom to the other.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
11
A covalent bond between two atoms is formed as a result of the
A)sharing of electrons.
B)loss of electrons from both atoms.
C)loss of a proton from one atom.
D)transfer of electrons from one atom to the other.
A)sharing of electrons.
B)loss of electrons from both atoms.
C)loss of a proton from one atom.
D)transfer of electrons from one atom to the other.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
12
You have a concentrated stock solution of 10 M NaOH and want to use it to produce a 150 mL solution of 3 M NaOH.What volume of water and stock solutions will you measure out to make this new solution?
A)135 mL of water; 15 mL of NaOH stock
B)115 mL of water; 35 mL of NaOH stock
C)100 mL of water; 50 mL of NaOH stock
D)105 mL of water; 45 mL of NaOH stock
A)135 mL of water; 15 mL of NaOH stock
B)115 mL of water; 35 mL of NaOH stock
C)100 mL of water; 50 mL of NaOH stock
D)105 mL of water; 45 mL of NaOH stock

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
13
Which subatomic particles contribute to the atomic mass for any given element?
A)protons
B)protons and neutrons
C)neutrons
D)protons and electrons
A)protons
B)protons and neutrons
C)neutrons
D)protons and electrons

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
14
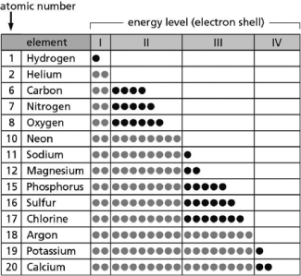 Table 2-14
Table 2-14-Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.On the basis of the information in the chart and what you know about atomic structure, which elements will form ions with a net charge of +1 in solution?
A)carbon; sulfur
B)helium; neon
C)sodium; potassium
D)magnesium; calcium

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
15
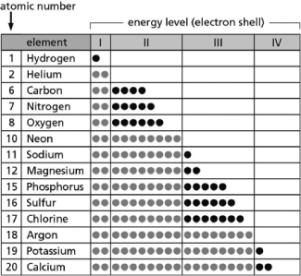 Table 2-14
Table 2-14-Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.On the basis of the information in the chart and what you know about atomic structure, which elements are chemically inert?
A)carbon; sulfur
B)helium; neon
C)sodium; potassium
D)magnesium; calcium

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following factors DO NOT influence the length of a covalent bond?
A)the tendency of atoms to fill the outer electron shells
B)the attractive forces between negatively charged electrons and positively charged nuclei
C)the repulsive forces between the positively charged nuclei
D)the minimization of repulsive forces between the two nuclei by the cloud of shared electrons
A)the tendency of atoms to fill the outer electron shells
B)the attractive forces between negatively charged electrons and positively charged nuclei
C)the repulsive forces between the positively charged nuclei
D)the minimization of repulsive forces between the two nuclei by the cloud of shared electrons

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
17
Polar covalent bonds are formed when the electrons in the bond are not shared equally between the two nuclei.Which one of these molecules contains polar bonds?
A)molecular oxygen
B)methane
C)propane
D)water
A)molecular oxygen
B)methane
C)propane
D)water

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
18
Figure 2-5 depicts the structure of carbon.Use the information in the diagram to choose the correct atomic number and atomic weight, respectively, for an atom of carbon. 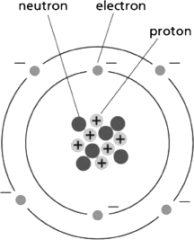 Figure 2-5
Figure 2-5
A)6; 12
B)12; 12
C)6; 18
D)12; 6
 Figure 2-5
Figure 2-5A)6; 12
B)12; 12
C)6; 18
D)12; 6

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
19
Double covalent bonds are both shorter and stronger than single covalent bonds, but they also limit the geometry of the molecule because they
A)create a new arrangement of electron shells.
B)change the reactivity of the bonded atoms.
C)limit the rotation of the bonded atoms.
D)prevent additional bonds from being formed with the bonded atoms.
A)create a new arrangement of electron shells.
B)change the reactivity of the bonded atoms.
C)limit the rotation of the bonded atoms.
D)prevent additional bonds from being formed with the bonded atoms.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
20
The first task you are assigned in your summer laboratory job is to prepare a concentrated NaOH stock solution.The molecular weight of NaOH is 40.How many grams of solid NaOH will you need to weigh out to obtain a 500 mL solution that has a concentration of 10 M?
A)800 g
B)200 g
C)400 g
D)160 g
A)800 g
B)200 g
C)400 g
D)160 g

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following monomer building blocks is necessary to assemble selectively permeable boundaries around and inside cells?
A)sugars
B)fatty acids
C)amino acids
D)nucleotides
A)sugars
B)fatty acids
C)amino acids
D)nucleotides

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
22
DNA and RNA are different types of nucleic acid polymer.Which of the following is true of DNA but NOT true of RNA?
A)It contains uracil.
B)It contains thymine.
C)It is single-stranded.
D)It has 5′-to-3′ directionality.
A)It contains uracil.
B)It contains thymine.
C)It is single-stranded.
D)It has 5′-to-3′ directionality.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
23
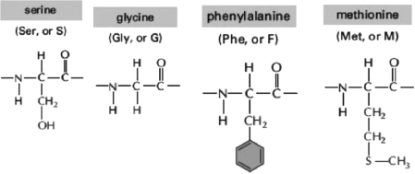 Figure 2-34
Figure 2-34-Oligosaccharides are short sugar polymers that can become covalently linked to proteins and lipids through condensation reactions.These modified proteins and lipids are called glycoproteins and glycolipids, respectively.Within a protein, which of the amino acids (shown in Figure 2-34) is the most probable target for this type of modification?
A)serine
B)glycine
C)phenylalanine
D)methionine

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
24
Choose the answer that best fits the following statement: Cholesterol is an essential component of biological membranes.Although it is much smaller than the typical phospholipids and glycolipids in the membrane, it is a/an __________ molecule, having both hydrophilic and hydrophobic regions.
A)polar
B)oxygen-containing
C)hydrophobic
D)amphipathic
A)polar
B)oxygen-containing
C)hydrophobic
D)amphipathic

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
25
Which combination of answers best completes the following statement: When atoms are held together by __________ __________, they are typically referred to as __________.
A)hydrogen bonds; molecules
B)ionic interactions; salts
C)ionic interactions; molecules
D)double bonds; nonpolar
A)hydrogen bonds; molecules
B)ionic interactions; salts
C)ionic interactions; molecules
D)double bonds; nonpolar

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
26
There is incredible chemical diversity even in the simplest of cells.A typical bacterial cell contains more than 6000 different types of molecules.From the list below, select the class of molecules with the largest number of different types.
A)nucleotides and precursors
B)sugars and precursors
C)amino acids and precursors
D)fatty acids and precursors
A)nucleotides and precursors
B)sugars and precursors
C)amino acids and precursors
D)fatty acids and precursors

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
27
Most types of molecules in the cell have asymmetric (chiral) carbons.Consequently there is the potential to have two different molecules that look much the same but are mirror images of each other and therefore not equivalent.These special types of isomers are called stereoisomers.Which of the four carbons circled in Figure 2-37 is the asymmetric carbon that determines whether the amino acid (threonine in this case) is a ᴅ or an ʟ stereoisomer? 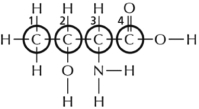 Figure 2-37
Figure 2-37
A)1
B)2
C)3
D)4
 Figure 2-37
Figure 2-37A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
28
Carbon atoms can form double bonds with other carbon atoms, nitrogen atoms, and oxygen atoms.Double bonds can have important consequences for biological molecules because they are __________ compared to single covalent bonds.
A)long
B)rigid with respect to rotation
C)weak
D)unstable
A)long
B)rigid with respect to rotation
C)weak
D)unstable

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
29
Cells contain buffers that help maintain a neutral pH.Which of the following statements is not relevant to how buffers work?
A)Buffers are mixtures of weak acids and bases.
B)Buffers can accept protons from acids.
C)Buffers can donate protons to bases.
D)Buffers catalyze oxidation-reduction reactions..
A)Buffers are mixtures of weak acids and bases.
B)Buffers can accept protons from acids.
C)Buffers can donate protons to bases.
D)Buffers catalyze oxidation-reduction reactions..

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
30
Although covalent bonds are 10-100 times stronger than noncovalent interactions, many biological processes depend upon the number and type of noncovalent interactions between molecules.Which of the noncovalent interactions below will contribute most to the strong and specific binding of two molecules, such as a pair of proteins?
A)electrostatic attractions
B)hydrogen bonds
C)hydrophobic interactions
D)van der Waals attractions
A)electrostatic attractions
B)hydrogen bonds
C)hydrophobic interactions
D)van der Waals attractions

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following are examples of isomers?
A)glucose and galactose
B)alanine and glycine
C)adenine and guanine
D)glycogen and cellulose
A)glucose and galactose
B)alanine and glycine
C)adenine and guanine
D)glycogen and cellulose

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
32
The amino acids glutamine and glutamic acid are shown in Figure 2-38.They differ only in the structure of part of their side chains (circled).At pH7, what type of interactions are possible for glutamic acid but not for glutamine? 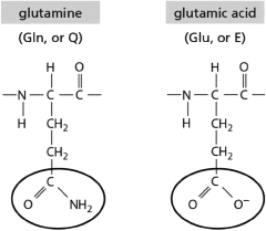 Figure 2-38
Figure 2-38
A)ionic bonds
B)hydrogen bonds
C)van der Waals interactions
D)covalent bonds
 Figure 2-38
Figure 2-38A)ionic bonds
B)hydrogen bonds
C)van der Waals interactions
D)covalent bonds

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
33
The pH of an aqueous solution is an indication of the concentration of available protons.However, you should not expect to find lone protons in solution; rather, the proton is added to a water molecule to form a/an __________ ion.
A)hydroxide
B)ammonium
C)chloride
D)hydronium
A)hydroxide
B)ammonium
C)chloride
D)hydronium

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
34
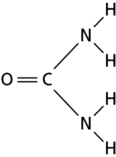 Figure 2-26
Figure 2-26-Larger molecules have hydrogen-bonding networks that contribute to specific, high-affinity binding.Smaller molecules such as urea can also form these networks.How many hydrogen bonds can urea (Figure 2-26) form if dissolved in water?
A)6
B)5
C)3
D)4

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
35
Many types of cells have stores of lipids in their cytoplasm, usually seen as fat droplets.What is the lipid most commonly found in these droplets?
A)cholesterol
B)palmitic acid
C)isoprene
D)triacylglycerol
A)cholesterol
B)palmitic acid
C)isoprene
D)triacylglycerol

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
36
Cells require one particular monosaccharide as a starting material to synthesize nucleotide building blocks.Which of the monosaccharides below fills this important role?
A)glucose
B)fructose
C)ribulose
D)ribose
A)glucose
B)fructose
C)ribulose
D)ribose

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
37
__________ play an important role in organizing lipid molecules with long hydrocarbon tails into biological membranes.
A)Hydrogen bonds
B)Ionic bonds
C)Hydrophobic forces
D)Van der Waals attractions
A)Hydrogen bonds
B)Ionic bonds
C)Hydrophobic forces
D)Van der Waals attractions

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
38
Aromatic carbon compounds such as benzene are planar and very stable.Double-bond character extends around the entire ring, which is why it is often drawn as a hexagon with a circle inside.This characteristic is caused by electron
A)resonance.
B)pairing.
C)partial charge.
D)stacking.
A)resonance.
B)pairing.
C)partial charge.
D)stacking.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following expressions accurately describes the calculation of pH?
A)pH = −log10[H+]
B)pH = log10[H+]
C)pH = −log2[H+]
D)pH = −log10[OH−]
A)pH = −log10[H+]
B)pH = log10[H+]
C)pH = −log2[H+]
D)pH = −log10[OH−]

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
40
Substances that release protons when they dissolve in water are acids.Which of the following household substance is acidic?
A)coffee
B)bleach
C)hand soap
D)water
A)coffee
B)bleach
C)hand soap
D)water

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
41
Match between columns
Premises:
Responses:
False
True
False
True
False
True

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
42
Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.Use the information in the table to fill in the blanks for A-E.There may be more than one answer for each. 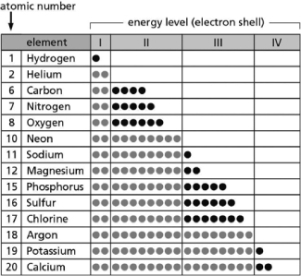
Table 2-14
A.__________ are chemically inert.
B.__________ form ions with a net charge of +1 in solution.
C.__________ form stable but reactive diatomic gases.
D.__________ form ions with a net charge of ?1 in solution.
E.__________ form ions with a net charge of +2 in solution.

Table 2-14
A.__________ are chemically inert.
B.__________ form ions with a net charge of +1 in solution.
C.__________ form stable but reactive diatomic gases.
D.__________ form ions with a net charge of ?1 in solution.
E.__________ form ions with a net charge of +2 in solution.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
43
Because there are four different monomer building blocks that can be used to assemble RNA polymers, the number of possible sequence combinations that can be created for an RNA molecule made of 100 nucleotides is
A)1004.
B)4100.
C)4 × 100.
D)100/4.
A)1004.
B)4100.
C)4 × 100.
D)100/4.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
44
The variety and arrangement of chemical groups on monomer subunits contribute to the conformation, reactivity, and surface of the macromolecule into which they become incorporated.What type of chemical group is circled on the nucleotide shown in Figure 2-41? 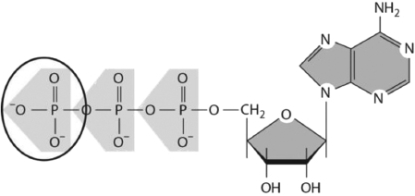 Figure 2-41
Figure 2-41
A)pyrophosphate
B)phosphoryl
C)carbonyl
D)carboxyl
 Figure 2-41
Figure 2-41A)pyrophosphate
B)phosphoryl
C)carbonyl
D)carboxyl

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
45
Match between columns
Premises:
Responses:
True
False
True
False
True
False

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
46
There are 20100 different possible sequence combinations for a protein chain with 100 amino acids.In addition to the amino acid sequence of the protein, what other factors INCREASE the potential for diversity in these macromolecules?
A)free rotation around single bonds during synthesis
B)noncovalent interactions sampled as protein folds
C)the directionality of amino acids being added
D)the planar nature of the peptide bond
A)free rotation around single bonds during synthesis
B)noncovalent interactions sampled as protein folds
C)the directionality of amino acids being added
D)the planar nature of the peptide bond

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
47
A.If 0.5 mole of glucose weighs 90 g, what is the molecular mass of glucose?
B.How much glucose do you have to add to water to produce 1 liter of a 0.25 M solution of glucose?
C.How many molecules are there in 1 mole of glucose?
B.How much glucose do you have to add to water to produce 1 liter of a 0.25 M solution of glucose?
C.How many molecules are there in 1 mole of glucose?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
48
Match between columns
Premises:
Responses:
True
False
True
False
True
False

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
49
A.What is the pH of pure water?
B.What concentration of hydronium ions does a solution of pH8 contain?
C.Complete the following reaction:
CH3COOH + H2O ↔ __________.
D.Will the reaction in (C) occur more readily (be driven to the right) if the pH of the solution is high?
B.What concentration of hydronium ions does a solution of pH8 contain?
C.Complete the following reaction:
CH3COOH + H2O ↔ __________.
D.Will the reaction in (C) occur more readily (be driven to the right) if the pH of the solution is high?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
50
You explain to a friend what you have learned about Avogadro's number.Your friend thinks the number is so large that he doubts there is even a mole of living cells on the Earth.You have recently heard that there are about 50 trillion (5 × 1013) human cells in each adult human body and that each human body carries more bacterial cells (the microbiome) than human cells, and the human population is approximately 7.6 billion (7.6 × 109).Armed with this information, you bet your friend $5 that there is more than a mole of cells on Earth.Write out the calculation that proves you are correct.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
51
Match between columns
Premises:
Responses:
True
False
True
False
True
False

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
52
Both DNA and RNA are synthesized by covalently linking a nucleoside triphosphate to the previous nucleotide, constantly adding to a growing chain.In the case of DNA, the new strand becomes part of a stable helix.The two strands are complementary in sequence and antiparallel in directionality.What is the principal force that holds these two strands together?
A)ionic interactions
B)hydrogen bonds
C)covalent bonds
D)van der Waals interactions
A)ionic interactions
B)hydrogen bonds
C)covalent bonds
D)van der Waals interactions

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
53
Match between columns
Premises:
Responses:
False
True
False
True
False
True
False
True

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
54
Match between columns
Premises:
Responses:
False
True
False
True
False
True

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
55
The relative strengths of covalent bonds and van der Waals interactions remain the same when tested in a vacuum or in water.However, this is not true of hydrogen bonds or ionic bonds, whose bond strengths are lowered considerably in the presence of water.Explain these observations.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
56
For each of the following sentences, fill in the blanks with the best word or phrase selected from the list below.Not all words or phrases will be used; each word or phrase should be used only once.
charge length polar
covalent molecule salt
double bond noncovalent single bond
ionic nonpolar weight
Whereas ionic bonds form a/an __________, covalent bonds between atoms form a/an __________.These covalent bonds have a characteristic bond __________ and become stronger and more rigid when two electrons are shared in a/an __________.Equal sharing of electrons yields a/an __________ covalent bond.If one atom participating in the bond has a stronger affinity for the electron, this produces a partial negative charge on one atom and a partial positive charge on the other.These __________ covalent bonds should not be confused with the weaker __________ bonds that are critical for the three-dimensional structure of biological molecules and for interactions between these molecules.
charge length polar
covalent molecule salt
double bond noncovalent single bond
ionic nonpolar weight
Whereas ionic bonds form a/an __________, covalent bonds between atoms form a/an __________.These covalent bonds have a characteristic bond __________ and become stronger and more rigid when two electrons are shared in a/an __________.Equal sharing of electrons yields a/an __________ covalent bond.If one atom participating in the bond has a stronger affinity for the electron, this produces a partial negative charge on one atom and a partial positive charge on the other.These __________ covalent bonds should not be confused with the weaker __________ bonds that are critical for the three-dimensional structure of biological molecules and for interactions between these molecules.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
57
Macromolecules in the cell can often interact transiently as a result of noncovalent interactions.These weak interactions also produce stable, highly specific interactions between molecules.Which of the factors below is the most significant in determining whether the interaction will be transient or stable?
A)the size of each molecule
B)the concentration of each molecule
C)the rate of synthesis
D)surface complementarity between molecules
A)the size of each molecule
B)the concentration of each molecule
C)the rate of synthesis
D)surface complementarity between molecules

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
58
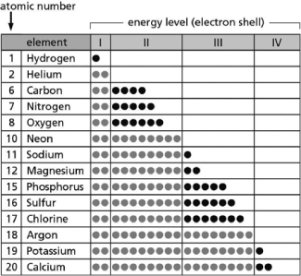 Table 2-14
Table 2-14-Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.On the basis of the information in the chart and what you know about atomic structure, which elements form stable but reactive diatomic gases?
A)nitrogen; oxygen
B)helium; neon
C)sodium; potassium
D)magnesium; calcium

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
59
A.In which scientific unit is the strength of a chemical bond usually expressed?
B.If 0.5 kilocalories of energy are required to break 6 × 1023 bonds of a particular type, what is the strength of this bond?
B.If 0.5 kilocalories of energy are required to break 6 × 1023 bonds of a particular type, what is the strength of this bond?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
60
Each nucleotide in DNA and RNA has an aromatic base.What is the principal force that keeps the bases in a polymer from interacting with water?
A)hydrophobic interactions
B)hydrogen bonds
C)covalent bonds
D)van der Waals interactions
A)hydrophobic interactions
B)hydrogen bonds
C)covalent bonds
D)van der Waals interactions

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
61
Eukaryotic cells have their DNA molecules inside their nuclei.However, to package all the DNA into such a small volume requires the cell to use specialized proteins called histones.Histones have amino acid sequences enriched for lysines and arginines.
A.What problem might a cell face in trying to package DNA into a small volume without histones, and how do these special packaging proteins alleviate the problem?
B.Lysine side chains are substrates for enzymes called acetylases.A diagram of an acetylated lysine side chain is shown in Figure 2-78.How do you think the acetylation of lysines in histone proteins will affect the ability of a histone to perform its role (refer to your answer in part A)?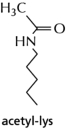
Figure 2-78
A.What problem might a cell face in trying to package DNA into a small volume without histones, and how do these special packaging proteins alleviate the problem?
B.Lysine side chains are substrates for enzymes called acetylases.A diagram of an acetylated lysine side chain is shown in Figure 2-78.How do you think the acetylation of lysines in histone proteins will affect the ability of a histone to perform its role (refer to your answer in part A)?

Figure 2-78

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
62
For each of the following sentences, fill in the blanks with the best word or phrase selected from the list below.Not all words or phrases will be used; each word or phrase should be used only once.
amino ionized polypeptides
α-carbon length protein
carbon noncovalent R group
carboxyl peptide bonds side chains
hydroxide
Proteins are __________ built from amino acids, which each have an amino group and a __________ group attached to the central __________.There are 20 possible __________ that differ in structure and are generally referred to as "R." In solutions of neutral pH, amino acids are __________, carrying both a positive and a negative charge.When a protein is made, amino acids are linked together through __________, which are formed by condensation reactions between the carboxyl end of the last amino acid and the __________ end of the next amino acid to be added to the growing chain.
amino ionized polypeptides
α-carbon length protein
carbon noncovalent R group
carboxyl peptide bonds side chains
hydroxide
Proteins are __________ built from amino acids, which each have an amino group and a __________ group attached to the central __________.There are 20 possible __________ that differ in structure and are generally referred to as "R." In solutions of neutral pH, amino acids are __________, carrying both a positive and a negative charge.When a protein is made, amino acids are linked together through __________, which are formed by condensation reactions between the carboxyl end of the last amino acid and the __________ end of the next amino acid to be added to the growing chain.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
63
A protein chain folds into its stable and unique three-dimensional structure, or conformation, by making many noncovalent bonds between different parts of the chain.Such noncovalent bonds are also critical for interactions with other proteins and cellular molecules.From the list provided, choose the class or classes of amino acids that are most important for the interactions detailed below.
A.forming ionic bonds with negatively charged DNA
B.forming hydrogen bonds to aid solubility in water
C.binding to another water-soluble protein
D.localizing an "integral membrane" protein that spans a lipid bilayer
E.tightly packing the hydrophobic interior core of a globular protein
acidic nonpolar
basic uncharged polar
A.forming ionic bonds with negatively charged DNA
B.forming hydrogen bonds to aid solubility in water
C.binding to another water-soluble protein
D.localizing an "integral membrane" protein that spans a lipid bilayer
E.tightly packing the hydrophobic interior core of a globular protein
acidic nonpolar
basic uncharged polar

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
64
Match between columns
Premises:
Responses:
False
True
False
True
False
True

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
65
A.Sketch three different ways in which three water molecules could be held together by hydrogen-bonding.
B.On a sketch of a single water molecule, indicate the distribution of positive and negative charge (using the symbols δ+ and δ−).
C.How many hydrogen bonds can a hydrogen atom in a water molecule form? How many hydrogen bonds can the oxygen atom in a water molecule form?
B.On a sketch of a single water molecule, indicate the distribution of positive and negative charge (using the symbols δ+ and δ−).
C.How many hydrogen bonds can a hydrogen atom in a water molecule form? How many hydrogen bonds can the oxygen atom in a water molecule form?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
66
The cell is able to harvest energy from various processes in order to generate ATP molecules.These ATPs represent a form of stored energy that can be used later to drive other important processes.Explain how the cell can convert the chemical energy stored in ATP to generate mechanical energy; for example, changing the shape of a protein.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
67
Selenium (Se) is an element required in the human body in trace amounts.Selenium is obtained through the diet and levels of selenium found in food depend greatly on the soil where it is grown.Once ingested and absorbed as selenate, it can become incorporated into a small number of polypeptides.These selenoproteins are formed when selenium replaces an element that is found in 2 of the 20 "standard" amino acids.Using your knowledge of atomic structure, the periodic table in Figure 2-7, and the structure of amino acids found in Panel 2-5, deduce which two amino acids may be converted to "seleno" amino acids and used to make selenoproteins.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
68
Silicon is an element that, like carbon, has four vacancies in its outer electron shell and therefore has the same bonding chemistry as carbon.Silicon is not found to any significant degree in the molecules found in living systems, however.Does this difference arise because elemental carbon is more abundant than silicon? What other explanations are there for the preferential selection of carbon over silicon as the basis for the molecules of life?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
69
You are trying to make a synthetic copy of a particular protein but accidentally join the amino acids together in exactly the reverse order.One of your classmates says the two proteins must be identical, and bets you $20 that your synthetic protein will have exactly the same biological activity as the original.After having read this chapter, you have no hesitation in staking your $20 that it will not.What particular feature of a polypeptide chain makes you sure your $20 is safe and that the project must be restarted?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
70
The amino acid histidine is often found in enzymes.Depending on the pH of its environment, sometimes histidine is neutral and at other times it acquires a proton and becomes positively charged.Consider an enzyme with a histidine side chain that is known to have an important role in the function of the enzyme.It is not clear whether this histidine is required in its protonated or its unprotonated state.To answer this question, you measure enzyme activity over a range of pH, with the results shown in Figure 2-70.Which form of histidine is necessary for the active enzyme? 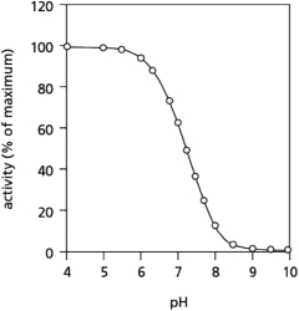
Figure 2-70

Figure 2-70

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
71
It is now a routine task to determine the exact order in which individual subunits have been linked together in polynucleotides (DNA) and polypeptides (proteins).However, it remains difficult to determine the arrangement of monomers in a polysaccharide.Explain why this is the case.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
72
Your lab director requests that you add new growth medium to the mammalian cell cultures before heading home from the lab on a Friday night.Unfortunately, you need to make fresh medium because all the premixed bottles of medium have been used.One of the ingredients you know you need to add is a mix of the essential amino acids (those that cannot be made by the cells, but are needed in proteins).On the shelf of dry chemicals you find the amino acids you need, and you mix them into your medium, along with all the other necessary nutrients, and replace the old medium with your new medium.On Sunday, you come in to the lab just to check on your cells and find that the cells have not grown.You are sure you made the medium correctly, but on checking you see that somebody wrote a note on the dry mixture of amino acids you used: "Note: this mixture contains only ᴅ-amino acids."
A.What is the meaning of the note and how does it explain the lack of cell growth in your culture?
B.Are there any organisms that could grow using this mixture? Justify your answer.
A.What is the meaning of the note and how does it explain the lack of cell growth in your culture?
B.Are there any organisms that could grow using this mixture? Justify your answer.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
73
You are trying to make a synthetic copy of a particular protein but accidentally join the amino acids together in exactly the reverse order.One of your classmates says the two proteins must be identical, and bets you $20 that your synthetic protein will have exactly the same biological activity as the original.After having read this chapter, you have no hesitation in staking your $20 that it will not.What particular feature of a polypeptide chain makes you sure your $20 is safe and that the project must be restarted?

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck
74
As a protein is made, the polypeptide is in an extended conformation, with every amino acid exposed to the aqueous environment.Although both polar and charged side chains can mix readily with water, this is not the case for nonpolar side chains.Explain how hydrophobic interactions may play a role in the early stages of protein folding, and have an influence on the final protein conformation.

Unlock Deck
Unlock for access to all 74 flashcards in this deck.
Unlock Deck
k this deck


