Exam 2: Chemical Components of Cells
Exam 1: Cells: The Fundamental Units of Life64 Questions
Exam 2: Chemical Components of Cells74 Questions
Exam 3: Energy, Catalysis, and Biosynthesis73 Questions
Exam 4: Protein Structure and Function71 Questions
Exam 5: DNA and Chromosomes69 Questions
Exam 6: DNA Replication and Repair61 Questions
Exam 7: From DNA to Protein62 Questions
Exam 8: Control of Gene Expression68 Questions
Exam 9: How Genes and Genomes Evolve60 Questions
Exam 10: Analyzing the Structure and Function of Genes59 Questions
Exam 11: Membrane Structure57 Questions
Exam 12: Transport Across Cell Membranes67 Questions
Exam 13: How Cells Obtain Energy From Food71 Questions
Exam 14: Energy Generation in Mitochondria and Chloroplasts72 Questions
Exam 15: Intracellular Compartments and Protein Transport55 Questions
Exam 16: Cell Signaling60 Questions
Exam 17: Cytoskeleton59 Questions
Exam 18: The Cell-Division Cycle67 Questions
Exam 19: Sexual Reproduction and the Power of Genetics61 Questions
Exam 20: Cell Communities: Tissues, Stem Cells, and Cancer57 Questions
Select questions type
Choose the answer that best fits the following statement: Cholesterol is an essential component of biological membranes.Although it is much smaller than the typical phospholipids and glycolipids in the membrane, it is a/an __________ molecule, having both hydrophilic and hydrophobic regions.
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
D
Most types of molecules in the cell have asymmetric (chiral) carbons.Consequently there is the potential to have two different molecules that look much the same but are mirror images of each other and therefore not equivalent.These special types of isomers are called stereoisomers.Which of the four carbons circled in Figure 2-37 is the asymmetric carbon that determines whether the amino acid (threonine in this case) is a ᴅ or an ʟ stereoisomer? 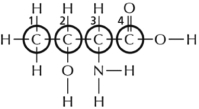 Figure 2-37
Figure 2-37
Free
(Multiple Choice)
4.8/5  (28)
(28)
Correct Answer:
C
The amino acid histidine is often found in enzymes.Depending on the pH of its environment, sometimes histidine is neutral and at other times it acquires a proton and becomes positively charged.Consider an enzyme with a histidine side chain that is known to have an important role in the function of the enzyme.It is not clear whether this histidine is required in its protonated or its unprotonated state.To answer this question, you measure enzyme activity over a range of pH, with the results shown in Figure 2-70.Which form of histidine is necessary for the active enzyme? 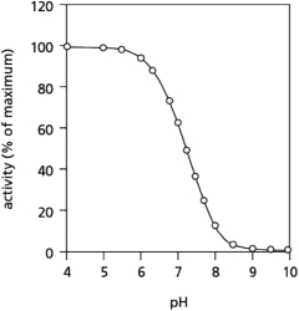 Figure 2-70
Figure 2-70
Free
(Essay)
4.8/5  (37)
(37)
Correct Answer:
Assuming that the change in enzyme activity is due to the change in the protonation state of histidine, the enzyme must require histidine in the protonated, charged state.The enzyme is active only at low, acidic pH, where the proton (or hydronium ion) concentration is high; thus, the loss of a proton from histidine will be disfavored so that histidine is likely to be protonated.
Indicate whether the statements below are TRUE or FALSE.If a statement is false, explain why it is false.
Correct Answer:
Premises:
Responses:
(Matching)
4.8/5  (31)
(31)
You explain to a friend what you have learned about Avogadro's number.Your friend thinks the number is so large that he doubts there is even a mole of living cells on the Earth.You have recently heard that there are about 50 trillion (5 × 1013) human cells in each adult human body and that each human body carries more bacterial cells (the microbiome) than human cells, and the human population is approximately 7.6 billion (7.6 × 109).Armed with this information, you bet your friend $5 that there is more than a mole of cells on Earth.Write out the calculation that proves you are correct.
(Essay)
4.7/5  (39)
(39)
Avogadro's number was established as the total number of units (atoms or molecules) in a mole, and a mole of any substance is X grams of it, where X is equal to the substance's molecular weight.A standard unit, the mole, allows scientists to calculate concentrations of materials dissolved in solutions.
Example: Sulfur has a molecular weight of 32.Therefore, 32 g of sulfur = 1 mole of sulfur = 6 × 1023 sulfur atoms.
How many moles and atoms, respectively, are there in 120 grams of sulfur?
(Multiple Choice)
5.0/5  (40)
(40)
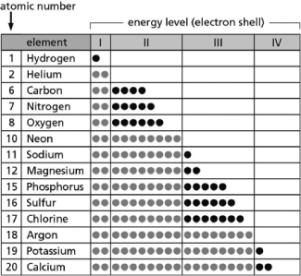 Table 2-14
-Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.On the basis of the information in the chart and what you know about atomic structure, which elements are chemically inert?
Table 2-14
-Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.On the basis of the information in the chart and what you know about atomic structure, which elements are chemically inert?
(Multiple Choice)
4.8/5  (26)
(26)
For each of the following sentences, fill in the blanks with the best word or phrase selected from the list below.Not all words or phrases will be used; each word or phrase should be used only once.
charge length polar covalent molecule salt double bond noncovalent single bond ionic nonpolar weight
Whereas ionic bonds form a/an __________, covalent bonds between atoms form a/an __________.These covalent bonds have a characteristic bond __________ and become stronger and more rigid when two electrons are shared in a/an __________.Equal sharing of electrons yields a/an __________ covalent bond.If one atom participating in the bond has a stronger affinity for the electron, this produces a partial negative charge on one atom and a partial positive charge on the other.These __________ covalent bonds should not be confused with the weaker __________ bonds that are critical for the three-dimensional structure of biological molecules and for interactions between these molecules.
(Essay)
4.8/5  (40)
(40)
Figure 2-5 depicts the structure of carbon.Use the information in the diagram to choose the correct atomic number and atomic weight, respectively, for an atom of carbon. 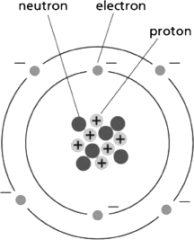 Figure 2-5
Figure 2-5
(Multiple Choice)
4.8/5  (29)
(29)
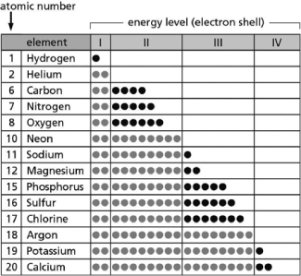 Table 2-14
-Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.On the basis of the information in the chart and what you know about atomic structure, which elements form stable but reactive diatomic gases?
Table 2-14
-Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.On the basis of the information in the chart and what you know about atomic structure, which elements form stable but reactive diatomic gases?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following monomer building blocks is necessary to assemble selectively permeable boundaries around and inside cells?
(Multiple Choice)
4.9/5  (40)
(40)
If the isotope 32S has 16 protons and 16 neutrons, how many protons, neutrons, and electrons will the isotope 35S have, respectively?
(Multiple Choice)
4.9/5  (32)
(32)
DNA and RNA are different types of nucleic acid polymer.Which of the following is true of DNA but NOT true of RNA?
(Multiple Choice)
4.7/5  (38)
(38)
The relative strengths of covalent bonds and van der Waals interactions remain the same when tested in a vacuum or in water.However, this is not true of hydrogen bonds or ionic bonds, whose bond strengths are lowered considerably in the presence of water.Explain these observations.
(Essay)
4.7/5  (35)
(35)
Substances that release protons when they dissolve in water are acids.Which of the following household substance is acidic?
(Multiple Choice)
4.8/5  (37)
(37)
Indicate whether the statements below are TRUE or FALSE.If a statement is false, explain why it is false.
Correct Answer:
Premises:
Responses:
(Matching)
5.0/5  (28)
(28)
Which subatomic particles contribute to the atomic mass for any given element?
(Multiple Choice)
4.8/5  (33)
(33)
Table 2-14 indicates the number and arrangement of electrons in the first four atomic electron shells for selected elements.Use the information in the table to fill in the blanks for A-E.There may be more than one answer for each. 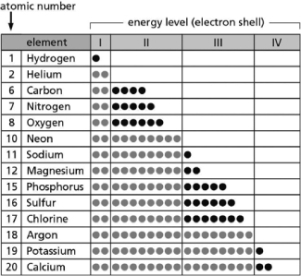 Table 2-14
A.__________ are chemically inert.
B.__________ form ions with a net charge of +1 in solution.
C.__________ form stable but reactive diatomic gases.
D.__________ form ions with a net charge of ?1 in solution.
E.__________ form ions with a net charge of +2 in solution.
Table 2-14
A.__________ are chemically inert.
B.__________ form ions with a net charge of +1 in solution.
C.__________ form stable but reactive diatomic gases.
D.__________ form ions with a net charge of ?1 in solution.
E.__________ form ions with a net charge of +2 in solution.
(Short Answer)
4.9/5  (33)
(33)
Aromatic carbon compounds such as benzene are planar and very stable.Double-bond character extends around the entire ring, which is why it is often drawn as a hexagon with a circle inside.This characteristic is caused by electron
(Multiple Choice)
4.8/5  (29)
(29)
Showing 1 - 20 of 74
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)