Deck 14: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/110
Play
Full screen (f)
Deck 14: Chemical Kinetics
1
The combustion of ethylene proceeds by the reaction C2H4 (g) + 3O2 (g) →2CO2 (g) + 2H2O (g)
When the rate of disappearance of O2 is 0.28 M s- 1, the rate of appearance of CO2 is _ _ M s- 1.
A) 0.84
B) 0.42
C) 0.56
D) 0.093
E) 0.19
When the rate of disappearance of O2 is 0.28 M s- 1, the rate of appearance of CO2 is _ _ M s- 1.
A) 0.84
B) 0.42
C) 0.56
D) 0.093
E) 0.19
0.19
2
The reaction A - B is first order in [A]. Consider the following data. ![<strong>The reaction A - B is first order in [A]. Consider the following data. The concentration of A is M after 40.0 s.</strong> A) 3.5 × 10<sup>-</sup><sup> </sup><sup>4</sup><sup> </sup> B) 0.025 C) 0.17 D) 1.2 E) 1.2 × 10<sup>-</sup><sup> </sup><sup>2</sup>](https://storage.examlex.com/TB1819/11ead62c_1f60_bcdf_ac95_811499ac21e7_TB1819_00.jpg)
The concentration of A is M after 40.0 s.
A) 3.5 × 10- 4
B) 0.025
C) 0.17
D) 1.2
E) 1.2 × 10- 2
![<strong>The reaction A - B is first order in [A]. Consider the following data. The concentration of A is M after 40.0 s.</strong> A) 3.5 × 10<sup>-</sup><sup> </sup><sup>4</sup><sup> </sup> B) 0.025 C) 0.17 D) 1.2 E) 1.2 × 10<sup>-</sup><sup> </sup><sup>2</sup>](https://storage.examlex.com/TB1819/11ead62c_1f60_bcdf_ac95_811499ac21e7_TB1819_00.jpg)
The concentration of A is M after 40.0 s.
A) 3.5 × 10- 4
B) 0.025
C) 0.17
D) 1.2
E) 1.2 × 10- 2
1.2 × 10- 2
3
In the energy profile of a reaction, the species that exists at the maximum on the curve is called the
)
A) enthalpy of reaction
B) activation energy
C) product
D) activated complex
E) atomic state
)
A) enthalpy of reaction
B) activation energy
C) product
D) activated complex
E) atomic state
activated complex
4
The rate law of the overall reaction ![<strong>The rate law of the overall reaction is rate = k[A]<sup>2</sup>. Which of the following will not increase the rate of the reaction?</strong> A) increasing the temperature of the reaction B) increasing the concentration of reactant A C) adding a catalyst for the reaction D) increasing the concentration of reactant B E) All of these will increase the rate.](https://storage.examlex.com/TB1819/11ead62c_1f5e_c10c_ac95_c96d8e062b9c_TB1819_11.jpg) is rate = k[A]2. Which of the following will not increase the rate of the reaction?
is rate = k[A]2. Which of the following will not increase the rate of the reaction?
A) increasing the temperature of the reaction
B) increasing the concentration of reactant A
C) adding a catalyst for the reaction
D) increasing the concentration of reactant B
E) All of these will increase the rate.
![<strong>The rate law of the overall reaction is rate = k[A]<sup>2</sup>. Which of the following will not increase the rate of the reaction?</strong> A) increasing the temperature of the reaction B) increasing the concentration of reactant A C) adding a catalyst for the reaction D) increasing the concentration of reactant B E) All of these will increase the rate.](https://storage.examlex.com/TB1819/11ead62c_1f5e_c10c_ac95_c96d8e062b9c_TB1819_11.jpg) is rate = k[A]2. Which of the following will not increase the rate of the reaction?
is rate = k[A]2. Which of the following will not increase the rate of the reaction?A) increasing the temperature of the reaction
B) increasing the concentration of reactant A
C) adding a catalyst for the reaction
D) increasing the concentration of reactant B
E) All of these will increase the rate.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
5
The primary source of the specificity of enzymes is .
A) their polarity, which matches that of their specific substrate
B) their locations within the cell
C) their shape, which relates to the lock- and- key model
D) their bonded transition metal, which is specific to the target substrate
E) their delocalized electron cloud
A) their polarity, which matches that of their specific substrate
B) their locations within the cell
C) their shape, which relates to the lock- and- key model
D) their bonded transition metal, which is specific to the target substrate
E) their delocalized electron cloud

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
6
Nitrogen fixation is a difficult process because .
A) there is so little nitrogen in the atmosphere
B) of the high polarity of nitrogen molecules preventing them from dissolving in biological fluids, such as those inside cells
C) nitrogen exists in the atmosphere primarily as its oxides which are very unreactive
D) of the extreme toxicity of nitrogen
E) nitrogen is very unreactive, largely due to its triple bond
A) there is so little nitrogen in the atmosphere
B) of the high polarity of nitrogen molecules preventing them from dissolving in biological fluids, such as those inside cells
C) nitrogen exists in the atmosphere primarily as its oxides which are very unreactive
D) of the extreme toxicity of nitrogen
E) nitrogen is very unreactive, largely due to its triple bond

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
7
A particular first- order reaction has a rate constant of 1.35 × 102 s- 1 at 25°C. What is the magnitude of k at 75°C if Ea = 85.6 kJ/mol?
A) 1.36 × 102
B) 3.85 × 106
C) 670
D) 1.93 × 104
E) 3.47 × 104
A) 1.36 × 102
B) 3.85 × 106
C) 670
D) 1.93 × 104
E) 3.47 × 104

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
8
The rate law of a reaction is rate = k[D][X]. The units of the rate constant are _ _.
A) L mol- 1s- 1
B) L2 mol- 2s- 1
C) mol2 L- 2s- 1
D) mol L- 1s- 1
E) mol L- 1s- 2
A) L mol- 1s- 1
B) L2 mol- 2s- 1
C) mol2 L- 2s- 1
D) mol L- 1s- 1
E) mol L- 1s- 2

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
9
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) →ClO3- (aq) + ClO2- (aq) + H2O (1)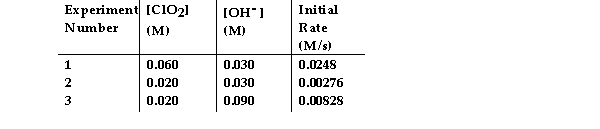
What is the order of the reaction with respect to ClO2?
A) 0
B) 2
C) 1
D) 3
E) 4
2 ClO2 (aq) + 2 OH- (aq) →ClO3- (aq) + ClO2- (aq) + H2O (1)

What is the order of the reaction with respect to ClO2?
A) 0
B) 2
C) 1
D) 3
E) 4

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
10
The reaction A - B is first order in [A]. Consider the following data. ![<strong>The reaction A - B is first order in [A]. Consider the following data. The half- life of this reaction is s.</strong> A) 0.14 B) 4.9 C) 0.97 D) 3.0 E) 7.1](https://storage.examlex.com/TB1819/11ead62c_1f5f_363d_ac95_83ed80bf8533_TB1819_00.jpg)
The half- life of this reaction is s.
A) 0.14
B) 4.9
C) 0.97
D) 3.0
E) 7.1
![<strong>The reaction A - B is first order in [A]. Consider the following data. The half- life of this reaction is s.</strong> A) 0.14 B) 4.9 C) 0.97 D) 3.0 E) 7.1](https://storage.examlex.com/TB1819/11ead62c_1f5f_363d_ac95_83ed80bf8533_TB1819_00.jpg)
The half- life of this reaction is s.
A) 0.14
B) 4.9
C) 0.97
D) 3.0
E) 7.1

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
11
As the temperature of a reaction is increased, the rate of the reaction increases because the
A) reactant molecules collide with greater energy per collision
B) reactant molecules collide less frequently and with greater energy per collision
C) activation energy is lowered
D) reactant molecules collide more frequently with less energy per collision
E) reactant molecules collide less frequently
A) reactant molecules collide with greater energy per collision
B) reactant molecules collide less frequently and with greater energy per collision
C) activation energy is lowered
D) reactant molecules collide more frequently with less energy per collision
E) reactant molecules collide less frequently

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
12
The reaction A - B is first order in [A]. Consider the following data. ![<strong>The reaction A - B is first order in [A]. Consider the following data. The rate constant for this reaction is s<sup>-</sup><sup> </sup><sup>1</sup>.</strong> A) 3.1 × 10<sup>-</sup><sup> </sup><sup>3</sup><sup> </sup> B) 0.013 C) 0.14 D) 3.0 E) 0.030](https://storage.examlex.com/TB1819/11ead62c_1f61_0b00_ac95_3fe68af3dba6_TB1819_00.jpg)
The rate constant for this reaction is s- 1.
A) 3.1 × 10- 3
B) 0.013
C) 0.14
D) 3.0
E) 0.030
![<strong>The reaction A - B is first order in [A]. Consider the following data. The rate constant for this reaction is s<sup>-</sup><sup> </sup><sup>1</sup>.</strong> A) 3.1 × 10<sup>-</sup><sup> </sup><sup>3</sup><sup> </sup> B) 0.013 C) 0.14 D) 3.0 E) 0.030](https://storage.examlex.com/TB1819/11ead62c_1f61_0b00_ac95_3fe68af3dba6_TB1819_00.jpg)
The rate constant for this reaction is s- 1.
A) 3.1 × 10- 3
B) 0.013
C) 0.14
D) 3.0
E) 0.030

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
13
SO2Cl2 decomposes in the gas phase by the reaction SO2Cl2 (g) →SO2 (g) + Cl2 (g)
The reaction is first order in SO2Cl2 and the rate constant is 3.0 × 10- 6 s- 1at 600 K. A vessel is charged with 2.4 atm of SO2Cl2 at 600 K. The partial pressure of SO2Cl2 at 3.0 × 105 s is
Atm)
A) 1.4 × 105
B) 0.76
C) 0.29
D) 2.2
E) 0.98
The reaction is first order in SO2Cl2 and the rate constant is 3.0 × 10- 6 s- 1at 600 K. A vessel is charged with 2.4 atm of SO2Cl2 at 600 K. The partial pressure of SO2Cl2 at 3.0 × 105 s is
Atm)
A) 1.4 × 105
B) 0.76
C) 0.29
D) 2.2
E) 0.98

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
14
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)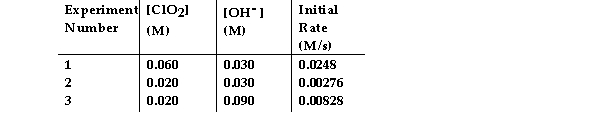
What is the order of the reaction with respect to OH- ?
A) 0
B) 1
C) 2
D) 3
E) 4
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)

What is the order of the reaction with respect to OH- ?
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
15
At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN):
CH3NC (g) → CH3CN (g)
The dependence of the rate constant on temperature is studied and the graph below is prepared from the results.
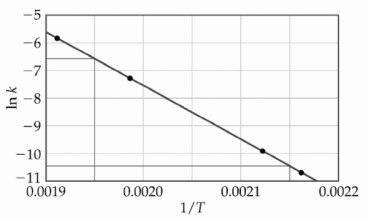
The energy of activation of this reaction is kJ/mol.
A) 1.6 × 105
B) 1.9 × 104
C) 4.4 × 10- 4
D) 160
E) 4.4 × 10- 7
CH3NC (g) → CH3CN (g)
The dependence of the rate constant on temperature is studied and the graph below is prepared from the results.

The energy of activation of this reaction is kJ/mol.
A) 1.6 × 105
B) 1.9 × 104
C) 4.4 × 10- 4
D) 160
E) 4.4 × 10- 7

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
16
SO2Cl2 decomposes in the gas phase by the reaction SO2Cl2 (g) →SO2 (g) + Cl2 (g)
The reaction is first order in SO2Cl2 and the rate constant is 3.0 × 10- 6 s- 1at 600 K. A vessel is charged with 3.3 atm of SO2Cl2 at 600 K. The partial pressure of SO2 at 3.0 × 105 s is _ _ atm.
A) 2.0
B) 3.7
C) 3.0
D) 2.1
E) 1.3
The reaction is first order in SO2Cl2 and the rate constant is 3.0 × 10- 6 s- 1at 600 K. A vessel is charged with 3.3 atm of SO2Cl2 at 600 K. The partial pressure of SO2 at 3.0 × 105 s is _ _ atm.
A) 2.0
B) 3.7
C) 3.0
D) 2.1
E) 1.3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
17
The isomerization of methylisonitrile to acetonitrile CH3NC (g) → CH3CN (g)
Is first order in CH3NC. The half life of the reaction is 1.60 × 105 s at 444 K. The rate constant when the initial [CH3NC] is 0.030 M is _ s- 1.
A) 2.08 × 10- 4
B) 4.80 × 103
C) 7.10 × 107
D) 2.31 × 105
E) 4.33 × 10- 6
Is first order in CH3NC. The half life of the reaction is 1.60 × 105 s at 444 K. The rate constant when the initial [CH3NC] is 0.030 M is _ s- 1.
A) 2.08 × 10- 4
B) 4.80 × 103
C) 7.10 × 107
D) 2.31 × 105
E) 4.33 × 10- 6

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
18
The elementary reaction
2NO2 (g) → 2NO (g) + O2 (g)
Is second order in NO2 and the rate constant at 501 K is 7.93 × 10- 3 M- 1s- 1. The reaction half- life at this temperature when [NO2]0 = 0.450 M is s.
A) 126
B) 0.011
C) 3.6 × 10- 3
D) 280
E) 87
2NO2 (g) → 2NO (g) + O2 (g)
Is second order in NO2 and the rate constant at 501 K is 7.93 × 10- 3 M- 1s- 1. The reaction half- life at this temperature when [NO2]0 = 0.450 M is s.
A) 126
B) 0.011
C) 3.6 × 10- 3
D) 280
E) 87

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
19
The reaction
2NO2 → 2NO + O2
Follows second- order kinetics. At 300°C, [NO2] drops from 0.0100- to 0.00650- M in 100 s. The rate constant for the reaction is _ M- 1s- 1.
A) 1.2
B) 0.096
C) 0.65
D) 0.54
E) 0.81
2NO2 → 2NO + O2
Follows second- order kinetics. At 300°C, [NO2] drops from 0.0100- to 0.00650- M in 100 s. The rate constant for the reaction is _ M- 1s- 1.
A) 1.2
B) 0.096
C) 0.65
D) 0.54
E) 0.81

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
20
Of the units below, are appropriate for a first- order reaction rate constant.
A) s- 1
B) mol/L
C) L mol- 1 s- 1
D) M s- 1
E) .M- 1 s- 1
A) s- 1
B) mol/L
C) L mol- 1 s- 1
D) M s- 1
E) .M- 1 s- 1

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
21
In general, as temperature goes up, reaction rate .
A) goes up if the reaction is endothermic
B) goes up if the reaction is exothermic
C) stays the same regardless of whether the reaction is exothermic or endothermic
D) goes up regardless of whether the reaction is exothermic or endothermic
E) stays the same if the reaction is first order
A) goes up if the reaction is endothermic
B) goes up if the reaction is exothermic
C) stays the same regardless of whether the reaction is exothermic or endothermic
D) goes up regardless of whether the reaction is exothermic or endothermic
E) stays the same if the reaction is first order

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
22
The combustion of ethylene proceeds by the reaction C2H4 (g) + 3O2 (g) → 2CO2 (g) + 2H2O (g)
When the rate of disappearance of O2 is 0.23 M s- 1, the rate of disappearance of C2H4 is
M s- 1.
A) 0.46
B) 0.15
C) 0.077
D) 0.35
E) 0.69
When the rate of disappearance of O2 is 0.23 M s- 1, the rate of disappearance of C2H4 is
M s- 1.
A) 0.46
B) 0.15
C) 0.077
D) 0.35
E) 0.69

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
23
Of the following, all are valid units for a reaction rate except _.
A) M/s
B) mol/L- hr
C) g/s
D) mol/hr
E) mol/L
A) M/s
B) mol/L- hr
C) g/s
D) mol/hr
E) mol/L

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
24
In the Arrhenius equation, k = Ae- Ea/RT Is the frequency factor.
A) R
B) A
C) Ea
D) e
E) k
A) R
B) A
C) Ea
D) e
E) k

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
25
The rate constant of a first- order process that has a half- life of 225 s is s- 1.
A) 3.08 × 10- 3
B) 0.693
C) 1.25
D) 12.5
E) 4.44 × 10- 3
A) 3.08 × 10- 3
B) 0.693
C) 1.25
D) 12.5
E) 4.44 × 10- 3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
26
are used in automotive catalytic converters.
A) Noble gases
B) Heterogeneous catalysts
C) Nonmetal oxides
D) Homogeneous catalysts
E) Enzymes
A) Noble gases
B) Heterogeneous catalysts
C) Nonmetal oxides
D) Homogeneous catalysts
E) Enzymes

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
27
The reaction A - B is first order in [A]. Consider the following data. ![<strong>The reaction A - B is first order in [A]. Consider the following data. The rate constant for this reaction is s<sup>-</sup><sup> </sup><sup>1</sup>.</strong> A) 0.46 B) 14 C) 3.0 × 10<sup>-</sup><sup> </sup><sup>2</sup><sup> </sup> D) 4.0 × 10<sup>2</sup><sup> </sup> E) 6.9 × 10<sup>-</sup><sup> </sup><sup>2</sup>](https://storage.examlex.com/TB1819/11ead62c_1f66_6237_ac95_4f8cb6bbc521_TB1819_00.jpg)
The rate constant for this reaction is s- 1.
A) 0.46
B) 14
C) 3.0 × 10- 2
D) 4.0 × 102
E) 6.9 × 10- 2
![<strong>The reaction A - B is first order in [A]. Consider the following data. The rate constant for this reaction is s<sup>-</sup><sup> </sup><sup>1</sup>.</strong> A) 0.46 B) 14 C) 3.0 × 10<sup>-</sup><sup> </sup><sup>2</sup><sup> </sup> D) 4.0 × 10<sup>2</sup><sup> </sup> E) 6.9 × 10<sup>-</sup><sup> </sup><sup>2</sup>](https://storage.examlex.com/TB1819/11ead62c_1f66_6237_ac95_4f8cb6bbc521_TB1819_00.jpg)
The rate constant for this reaction is s- 1.
A) 0.46
B) 14
C) 3.0 × 10- 2
D) 4.0 × 102
E) 6.9 × 10- 2

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
28
Of the following, will lower the activation energy for a reaction.
A) raising the temperature of the reaction
B) increasing the concentrations of reactants
C) adding a catalyst for the reaction
D) removing products as the reaction proceeds
E) increasing the pressure
A) raising the temperature of the reaction
B) increasing the concentrations of reactants
C) adding a catalyst for the reaction
D) removing products as the reaction proceeds
E) increasing the pressure

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
29
The reaction
CH3- N≡C - CH3- C≡N
Is a first- order reaction. At 230.3°C, k = 6.29 × 10- 4 s- 1. If [CH3- N≡C] is 1.00 × 10- 3 initially, [CH3- N≡C] is after 1.000 × 103 s.
A) 2.34 × 10- 4
B) 4.27 × 10- 3
C) 1.00 × 10- 6
D) 5.33 × 10- 4
E) 1.88 × 10- 3
CH3- N≡C - CH3- C≡N
Is a first- order reaction. At 230.3°C, k = 6.29 × 10- 4 s- 1. If [CH3- N≡C] is 1.00 × 10- 3 initially, [CH3- N≡C] is after 1.000 × 103 s.
A) 2.34 × 10- 4
B) 4.27 × 10- 3
C) 1.00 × 10- 6
D) 5.33 × 10- 4
E) 1.88 × 10- 3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
30
The rate of disappearance of HBr in the gas phase reaction 2HBr (g) → H2 (g) + Br2 (g)
Is 0.190 M s- 1 at 150°C. The rate of reaction is M s- 1.
A) 2.63
B) 0.086
C) 0.0361
D) 0.095
E) 0.380
Is 0.190 M s- 1 at 150°C. The rate of reaction is M s- 1.
A) 2.63
B) 0.086
C) 0.0361
D) 0.095
E) 0.380

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
31
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)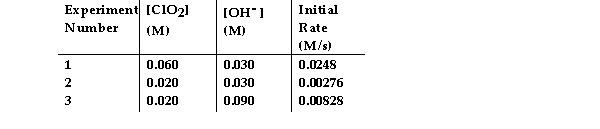
What is the overall order of the reaction?
A) 4
B) 2
C) 1
D) 3
E) 0
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1)

What is the overall order of the reaction?
A) 4
B) 2
C) 1
D) 3
E) 0

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
32
The rate of disappearance of HBr in the gas phase reaction 2HBr (g) →H2 (g) + Br2 (g)
Is 0.301 M s- 1 at 150°C. The rate of appearance of Br2 is M s- 1.
A) 0.549
B) 1.66
C) 0.602
D) 0.151
E) 0.0906
Is 0.301 M s- 1 at 150°C. The rate of appearance of Br2 is M s- 1.
A) 0.549
B) 1.66
C) 0.602
D) 0.151
E) 0.0906

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
33
The data in the table below were obtained for the reaction:
A + B → P
The magnitude of the rate constant is .
A) 38.0
B) 0.278
C) 13.2
D) 2.21
E) 42.0
A + B → P

The magnitude of the rate constant is .
A) 38.0
B) 0.278
C) 13.2
D) 2.21
E) 42.0

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
34
At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN):
CH3NC (g) → CH33CN (g)
The reaction is first order in methylisonitrile. The attached graph shows data for the reaction obtained at 198.9°C.
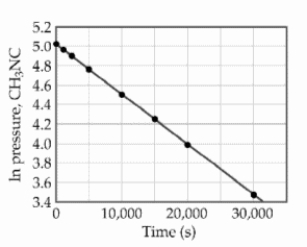
The rate constant for the reaction is s- 1.
A) +5.2 × 10- 5
B) +6.2
C) +1.9 × 104
D) - 5.2 × 10- 5
E) - 1.9 × 104
CH3NC (g) → CH33CN (g)
The reaction is first order in methylisonitrile. The attached graph shows data for the reaction obtained at 198.9°C.

The rate constant for the reaction is s- 1.
A) +5.2 × 10- 5
B) +6.2
C) +1.9 × 104
D) - 5.2 × 10- 5
E) - 1.9 × 104

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
35
The reaction A (aq) - B (aq) is first order in [A]. A solution is prepared with [A] = 1.22 M. The following data are obtained as the reaction proceeds:
![<strong>The reaction A (aq) - B (aq) is first order in [A]. A solution is prepared with [A] = 1.22 M. The following data are obtained as the reaction proceeds: The rate constant for this reaction is s<sup>-</sup><sup> </sup><sup>1</sup>.</strong> A) 0.17 B) - 0.12 C) 0.23 D) 1.0 E) 0.12](https://storage.examlex.com/TB1819/11ead62c_1f64_6665_ac95_730d29e995f8_TB1819_00.jpg)
The rate constant for this reaction is s- 1.
A) 0.17
B) - 0.12
C) 0.23
D) 1.0
E) 0.12
![<strong>The reaction A (aq) - B (aq) is first order in [A]. A solution is prepared with [A] = 1.22 M. The following data are obtained as the reaction proceeds: The rate constant for this reaction is s<sup>-</sup><sup> </sup><sup>1</sup>.</strong> A) 0.17 B) - 0.12 C) 0.23 D) 1.0 E) 0.12](https://storage.examlex.com/TB1819/11ead62c_1f64_6665_ac95_730d29e995f8_TB1819_00.jpg)
The rate constant for this reaction is s- 1.
A) 0.17
B) - 0.12
C) 0.23
D) 1.0
E) 0.12

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
36
The half- life of a first- order reaction .
A) is constant
B) does not depend on the initial reactant concentration
C) can be calculated from the reaction rate constant
D) is the time necessary for the reactant concentration to drop to half its original value
E) All of the above are correct.
A) is constant
B) does not depend on the initial reactant concentration
C) can be calculated from the reaction rate constant
D) is the time necessary for the reactant concentration to drop to half its original value
E) All of the above are correct.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
37
The isomerization of methylisonitrile to acetonitrile CH3NC (g) → CH3CN (g)
Is first order in CH3NC. The rate constant for the reaction is 9.45 × 10- 5 s- 1 at 478 K. The half- life of the reaction when the initial [CH3NC] is 0.030 M is s.
A) 3.53E × 105
B) 1.36 × 10- 4
C) 7.33 × 103
D) 1.06 × 104
E) 5.29 × 103
Is first order in CH3NC. The rate constant for the reaction is 9.45 × 10- 5 s- 1 at 478 K. The half- life of the reaction when the initial [CH3NC] is 0.030 M is s.
A) 3.53E × 105
B) 1.36 × 10- 4
C) 7.33 × 103
D) 1.06 × 104
E) 5.29 × 103

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
38
A particular first- order reaction has a rate constant of 1.35 × 102 s- 1 at 25°C. What is the magnitude of k at 95°C if Ea = 55.5 kJ/mol?
A) 4.33 × 1087
B) 1.36 × 102
C) 576
D) 2.85 × 104
E) 9.60 × 103
A) 4.33 × 1087
B) 1.36 × 102
C) 576
D) 2.85 × 104
E) 9.60 × 103

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
39
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) →ClO3- (aq) + ClO2- (aq) + H2O (1)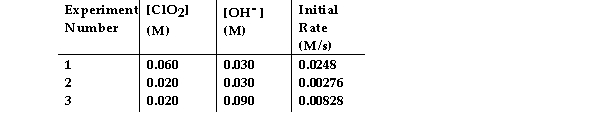
What is the magnitude of the rate constant for the reaction?
A) 4.6
B) 115
C) 230
D) 713
E) 1.15 × 104
2 ClO2 (aq) + 2 OH- (aq) →ClO3- (aq) + ClO2- (aq) + H2O (1)

What is the magnitude of the rate constant for the reaction?
A) 4.6
B) 115
C) 230
D) 713
E) 1.15 × 104

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
40
One difference between first- and second- order reactions is that .
A) the rate of a first- order reaction does not depend on reactant concentrations; the rate of a second- order reaction does depend on reactant concentrations
B) a first- order reaction can be catalyzed; a second- order reaction cannot be catalyzed
C) the rate of a first- order reaction depends on reactant concentrations; the rate of a second- order reaction does not depend on reactant concentrations
D) the half- life of a first- order reaction depends on [A]0; the half- life of a second- order reaction does not depend on [A]0
E) the half- life of a first- order reaction does not depend on [A]0; the half- life of a second- order reaction does depend on [A]0
A) the rate of a first- order reaction does not depend on reactant concentrations; the rate of a second- order reaction does depend on reactant concentrations
B) a first- order reaction can be catalyzed; a second- order reaction cannot be catalyzed
C) the rate of a first- order reaction depends on reactant concentrations; the rate of a second- order reaction does not depend on reactant concentrations
D) the half- life of a first- order reaction depends on [A]0; the half- life of a second- order reaction does not depend on [A]0
E) the half- life of a first- order reaction does not depend on [A]0; the half- life of a second- order reaction does depend on [A]0

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
41
The active site of nitrogenase is a cofactor that contains two transition metals. These transition metals are .
A) Os and Ir
B) Fe and Zn
C) Mn and V
D) Fe and Mo
E) Cr and Mg
A) Os and Ir
B) Fe and Zn
C) Mn and V
D) Fe and Mo
E) Cr and Mg

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
42
Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction:
2NO2 → 2NO + O2
In a particular experiment at 300°C, [NO2] drops from 0.0100 to 0.00650 M in 100 s. The rate of disappearance of NO2 for this period is _ M/s.
A) 0.35
B) 1.8 × 10- 3
C) 3.5 × 10- 3
D) 7.0 × 10- 3
E) 3.5 × 10- 5
2NO2 → 2NO + O2
In a particular experiment at 300°C, [NO2] drops from 0.0100 to 0.00650 M in 100 s. The rate of disappearance of NO2 for this period is _ M/s.
A) 0.35
B) 1.8 × 10- 3
C) 3.5 × 10- 3
D) 7.0 × 10- 3
E) 3.5 × 10- 5

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
43
For the reaction aA + Bb → cC + dD the rate law is .

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
44
The relationship of absorbed light to the concentration of the substance absorbing the light is governed by .

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
45
The number of molecules that participate as reactants defines the of the reaction.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
46
The rate of a reaction depends on _.
A) collision frequency
B) collision orientation
C) collision energy
D) all of the above
E) none of the above
A) collision frequency
B) collision orientation
C) collision energy
D) all of the above
E) none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
47
The rate law for a reaction is
Rate = k [A][B]2
Which one of the following statements is false?
A) The reaction is second order overall.
B) The reaction is second order in B.
C) k is the reaction rate constant
D) If [B] is doubled, the reaction rate will increase by a factor of 4.
E) The reaction is first order in A.
Rate = k [A][B]2
Which one of the following statements is false?
A) The reaction is second order overall.
B) The reaction is second order in B.
C) k is the reaction rate constant
D) If [B] is doubled, the reaction rate will increase by a factor of 4.
E) The reaction is first order in A.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
48
A burning splint will burn more vigorously in pure oxygen than in air because
A) nitrogen is a reactant in combustion and its low concentration in pure oxygen catalyzes the combustion.
B) nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature.
C) oxygen is a reactant in combustion and concentration of oxygen is higher in pure oxygen than is in air.
D) oxygen is a catalyst for combustion.
E) oxygen is a product of combustion.
A) nitrogen is a reactant in combustion and its low concentration in pure oxygen catalyzes the combustion.
B) nitrogen is a product of combustion and the system reaches equilibrium at a lower temperature.
C) oxygen is a reactant in combustion and concentration of oxygen is higher in pure oxygen than is in air.
D) oxygen is a catalyst for combustion.
E) oxygen is a product of combustion.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
49
The minimum energy to initiate a chemical reaction is the .

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
50
Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?
A)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://storage.examlex.com/TB1819/11ead62c_1f67_9ab9_ac95_9d7b618e93eb_TB1819_00.jpg)
B)![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://storage.examlex.com/TB1819/11ead62c_1f67_9aba_ac95_e3a983bb27d3_TB1819_00.jpg)
C)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://storage.examlex.com/TB1819/11ead62c_1f67_c1cb_ac95_4170f0a2229a_TB1819_00.jpg)
D)![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://storage.examlex.com/TB1819/11ead62c_1f67_e8dc_ac95_d18a13560d17_TB1819_00.jpg)
E)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://storage.examlex.com/TB1819/11ead62c_1f67_e8dd_ac95_7fb4860c8ee7_TB1819_00.jpg)
A)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://storage.examlex.com/TB1819/11ead62c_1f67_9ab9_ac95_9d7b618e93eb_TB1819_00.jpg)
B)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://storage.examlex.com/TB1819/11ead62c_1f67_9aba_ac95_e3a983bb27d3_TB1819_00.jpg)
C)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://storage.examlex.com/TB1819/11ead62c_1f67_c1cb_ac95_4170f0a2229a_TB1819_00.jpg)
D)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://storage.examlex.com/TB1819/11ead62c_1f67_e8dc_ac95_d18a13560d17_TB1819_00.jpg)
E)
![<strong>Which one of the following graphs shows the correct relationship between concentration and time for a reaction that is second order in [A]?</strong> A) B) C) D) E)](https://storage.examlex.com/TB1819/11ead62c_1f67_e8dd_ac95_7fb4860c8ee7_TB1819_00.jpg)

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
51
The following reaction is second order in [A] and the rate constant is 0.039 M- 1s- 1:
A - B
The concentration of A was 0.30 M at 23s. The initial concentration of A was _ M.
A) 2.4
B) 1.2 × 10- 2
C) 3.7
D) 0.41
E) 0.27
A - B
The concentration of A was 0.30 M at 23s. The initial concentration of A was _ M.
A) 2.4
B) 1.2 × 10- 2
C) 3.7
D) 0.41
E) 0.27

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
52
The overall reactions and rate laws for several reactions are given below. Of these, only _ could represent an elementary step.
A)
B)
C)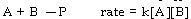
D)
E)
A)

B)

C)
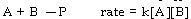
D)

E)


Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
53
The decomposition of N2O5 in solution in carbon tetrachloride proceeds via the reaction 2N2O5 (soln) → 4NO2 (soln) + O2 (soln)
The reaction is first order and has a rate constant of 4.82 × 10- 3 s- 1 at 64°C. If the reaction is initiated with 0.058 mol in a 1.00- L vessel, how many moles remain after 151 s?
A) 0.055
B) 2.0 × 103
C) 0.028
D) 0.060
E) 12
The reaction is first order and has a rate constant of 4.82 × 10- 3 s- 1 at 64°C. If the reaction is initiated with 0.058 mol in a 1.00- L vessel, how many moles remain after 151 s?
A) 0.055
B) 2.0 × 103
C) 0.028
D) 0.060
E) 12

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
54
The mechanism for formation of the product X is:
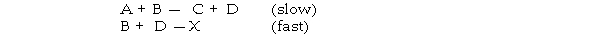
The intermediate reactant in the reaction is .
A) A
B) B
C) C
D) D
E) X
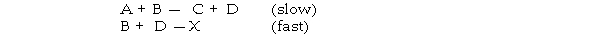
The intermediate reactant in the reaction is .
A) A
B) B
C) C
D) D
E) X

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
55
A catalyst can increase the rate of a reaction .
A) by providing an alternative pathway with a lower activation energy
B) by lowering the overall activation energy (Ea) of the reaction
C) by lowering the activation energy of the reverse reaction
D) by changing the value of the frequency factor (A)
E) All of these are ways that a catalyst might act to increase the rate of reaction.
A) by providing an alternative pathway with a lower activation energy
B) by lowering the overall activation energy (Ea) of the reaction
C) by lowering the activation energy of the reverse reaction
D) by changing the value of the frequency factor (A)
E) All of these are ways that a catalyst might act to increase the rate of reaction.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
56
The enzyme nitrogenase converts _ _ into _.
A) nitrogen oxides, N2 and O2
B) nitroglycerine, nitric acid, and glycerine
C) ammonia, urea
D) CO and unburned hydrocarbons, H2O and CO2
E) nitrogen, ammonia
A) nitrogen oxides, N2 and O2
B) nitroglycerine, nitric acid, and glycerine
C) ammonia, urea
D) CO and unburned hydrocarbons, H2O and CO2
E) nitrogen, ammonia

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
57
Which energy difference in the energy profile below corresponds to the activation energy for the forward reaction?
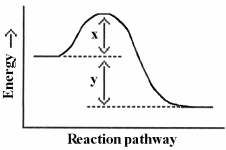
A) x
B) y
C) x - y
D) x + y
E) y - x

A) x
B) y
C) x - y
D) x + y
E) y - x

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
58
The earth's ozone layer is located in the .

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
59
Reaction rate data obey an equation devised by _ .

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
60
For the elementary reaction ![<strong>For the elementary reaction the molecularity of the reaction is , and the rate law is rate = .</strong> A) 2, k[NO<sub>3</sub>][CO]/[NO<sub>2</sub>][CO<sub>2</sub>] B) 2, k[NO<sub>2</sub>][CO<sub>2</sub>] C) 4, k[NO<sub>3</sub>][CO][NO<sub>2</sub>][CO<sub>2</sub>] D) 4, k[NO<sub>2</sub>][CO<sub>2</sub>]/[NO<sub>3</sub>][CO] E) 2, k[NO<sub>3</sub>][CO]](https://storage.examlex.com/TB1819/11ead62c_1f69_4871_ac95_2bd23521ed09_TB1819_00.jpg) the molecularity of the reaction is , and the rate law is rate = .
the molecularity of the reaction is , and the rate law is rate = .
A) 2, k[NO3][CO]/[NO2][CO2]
B) 2, k[NO2][CO2]
C) 4, k[NO3][CO][NO2][CO2]
D) 4, k[NO2][CO2]/[NO3][CO]
E) 2, k[NO3][CO]
![<strong>For the elementary reaction the molecularity of the reaction is , and the rate law is rate = .</strong> A) 2, k[NO<sub>3</sub>][CO]/[NO<sub>2</sub>][CO<sub>2</sub>] B) 2, k[NO<sub>2</sub>][CO<sub>2</sub>] C) 4, k[NO<sub>3</sub>][CO][NO<sub>2</sub>][CO<sub>2</sub>] D) 4, k[NO<sub>2</sub>][CO<sub>2</sub>]/[NO<sub>3</sub>][CO] E) 2, k[NO<sub>3</sub>][CO]](https://storage.examlex.com/TB1819/11ead62c_1f69_4871_ac95_2bd23521ed09_TB1819_00.jpg) the molecularity of the reaction is , and the rate law is rate = .
the molecularity of the reaction is , and the rate law is rate = .A) 2, k[NO3][CO]/[NO2][CO2]
B) 2, k[NO2][CO2]
C) 4, k[NO3][CO][NO2][CO2]
D) 4, k[NO2][CO2]/[NO3][CO]
E) 2, k[NO3][CO]

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
61
The average rate of disappearance of I- between 400 s and 800 s is M/s.
A) 1.4 × 10- 5
B) 3.6 × 104
C) 5.8 × 10- 5
D) 2.6 × 10- 4
E) 2.8 × 10- 5
A) 1.4 × 10- 5
B) 3.6 × 104
C) 5.8 × 10- 5
D) 2.6 × 10- 4
E) 2.8 × 10- 5

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
62
If the rate law for the reaction ![<strong>If the rate law for the reaction is first order in A and second order in B, then the rate law is rate = .</strong> A) k[A]<sup>2</sup>[B]<sup>2</sup><sup> </sup> B) k[A][B] C) k[A][B]<sup>2</sup><sup> </sup> D) k[A]<sup>2</sup>[B]<sup>3</sup><sup> </sup> E) k[A]<sup>2</sup>[B]](https://storage.examlex.com/TB1819/11ead62c_1f6a_0bc2_ac95_7129822a4a55_TB1819_00.jpg) is first order in A and second order in B, then the rate law is rate = .
is first order in A and second order in B, then the rate law is rate = .
A) k[A]2[B]2
B) k[A][B]
C) k[A][B]2
D) k[A]2[B]3
E) k[A]2[B]
![<strong>If the rate law for the reaction is first order in A and second order in B, then the rate law is rate = .</strong> A) k[A]<sup>2</sup>[B]<sup>2</sup><sup> </sup> B) k[A][B] C) k[A][B]<sup>2</sup><sup> </sup> D) k[A]<sup>2</sup>[B]<sup>3</sup><sup> </sup> E) k[A]<sup>2</sup>[B]](https://storage.examlex.com/TB1819/11ead62c_1f6a_0bc2_ac95_7129822a4a55_TB1819_00.jpg) is first order in A and second order in B, then the rate law is rate = .
is first order in A and second order in B, then the rate law is rate = .A) k[A]2[B]2
B) k[A][B]
C) k[A][B]2
D) k[A]2[B]3
E) k[A]2[B]

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
63
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g). The following data are obtained for [A] as the reaction proceeds: ![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of disappearance of A between 10 s and 20 s is mol/s.</strong> A) 1.1 × 10<sup>-</sup><sup> </sup><sup>3</sup> B) 4.4 × 10<sup>-</sup><sup> </sup><sup>3</sup> C) 9.90 × 10<sup>- </sup><sup>3</sup> D) 454 E) 2.2 × 10<sup>- </sup><sup>3</sup>](https://storage.examlex.com/TB1819/11ead62c_1f6a_a804_ac95_31c6da222cb4_TB1819_00.jpg)
The average rate of disappearance of A between 10 s and 20 s is mol/s.
A) 1.1 × 10- 3
B) 4.4 × 10- 3
C) 9.90 × 10- 3
D) 454
E) 2.2 × 10- 3
![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of disappearance of A between 10 s and 20 s is mol/s.</strong> A) 1.1 × 10<sup>-</sup><sup> </sup><sup>3</sup> B) 4.4 × 10<sup>-</sup><sup> </sup><sup>3</sup> C) 9.90 × 10<sup>- </sup><sup>3</sup> D) 454 E) 2.2 × 10<sup>- </sup><sup>3</sup>](https://storage.examlex.com/TB1819/11ead62c_1f6a_a804_ac95_31c6da222cb4_TB1819_00.jpg)
The average rate of disappearance of A between 10 s and 20 s is mol/s.
A) 1.1 × 10- 3
B) 4.4 × 10- 3
C) 9.90 × 10- 3
D) 454
E) 2.2 × 10- 3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
64
The concentration of S2O82- remaining at 400 s is _ M.
A) +0.057
B) - 0.007
C) +0.015
D) +0.035
E) +0.045
A) +0.057
B) - 0.007
C) +0.015
D) +0.035
E) +0.045

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
65
The reaction below is first order in [H2O2]:
2H2O2 (l) - 2H2O (l) + O2 (g)
A solution originally at 0.600 M H2O2 is found to be 0.075 M after 54 min. The half- life for this reaction is min.
A) 28
B) 6.8
C) 18
D) 14
E) 54
2H2O2 (l) - 2H2O (l) + O2 (g)
A solution originally at 0.600 M H2O2 is found to be 0.075 M after 54 min. The half- life for this reaction is min.
A) 28
B) 6.8
C) 18
D) 14
E) 54

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
66
A second- order reaction has a half- life of 18 s when the initial concentration of reactant is 0.71 M. The rate constant for this reaction is M- 1s- 1.
A) 3.8 × 10- 2
B) 7.8 × 10- 2
C) 18
D) 1.3
E) 2.0 × 10- 2
A) 3.8 × 10- 2
B) 7.8 × 10- 2
C) 18
D) 1.3
E) 2.0 × 10- 2

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
67
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g). The following data are obtained for [A] as the reaction proceeds: ![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g). The following data are obtained for [A] as the reaction proceeds: How many moles of B are present at 10 s?</strong> A) 0.220 B) 0.110 C) 1.4 × 10<sup>-</sup><sup> </sup><sup>3</sup><sup> </sup> D) 0.014 E) 0.011](https://storage.examlex.com/TB1819/11ead62c_1f6a_59e3_ac95_59e6cc273c18_TB1819_00.jpg)
How many moles of B are present at 10 s?
A) 0.220
B) 0.110
C) 1.4 × 10- 3
D) 0.014
E) 0.011
![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g). The following data are obtained for [A] as the reaction proceeds: How many moles of B are present at 10 s?</strong> A) 0.220 B) 0.110 C) 1.4 × 10<sup>-</sup><sup> </sup><sup>3</sup><sup> </sup> D) 0.014 E) 0.011](https://storage.examlex.com/TB1819/11ead62c_1f6a_59e3_ac95_59e6cc273c18_TB1819_00.jpg)
How many moles of B are present at 10 s?
A) 0.220
B) 0.110
C) 1.4 × 10- 3
D) 0.014
E) 0.011

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
68
The rate constant for a particular second- order reaction is 0.47 M- 1s- 1. If the initial concentration of reactant is 0.25 mol/L, it takes s for the concentration to decrease to 0.13 mol/L.
A) 1.7
B) 1.4
C) 7.9
D) 0.13
E) 3.7
A) 1.7
B) 1.4
C) 7.9
D) 0.13
E) 3.7

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
69
The concentration of S2O82- remaining at 1600 s is M.
A) 0.014
B) 0.043
C) 0.029
D) 0.064
E) 0.036
A) 0.014
B) 0.043
C) 0.029
D) 0.064
E) 0.036

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
70
The kinetics of the reaction below were studied and it was determined that the reaction rate increased by a factor of 9 when the concentration of B was tripled. The reaction is order in B.
A + B → P
A) zero
B) first
C) second
D) third
E) one- half
A + B → P
A) zero
B) first
C) second
D) third
E) one- half

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
71
If a rate law is second order (reactant) , doubling the reactant _ _ the reaction rate.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
72
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g). The following data are obtained for [A] as the reaction proceeds: ![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of appearance of B between 20 s and 30 s is mol/s.</strong> A) +5.0 × 10<sup>-</sup><sup> </sup><sup>4</sup> B) +1.5 × 10<sup>-</sup><sup> </sup><sup>3</sup> C) - 1.5 × 10<sup>-</sup><sup> </sup><sup>3</sup> D) - 7.3 × 10<sup>-</sup><sup> </sup><sup>3</sup> E) +7.3 × 10<sup>-</sup><sup> </sup><sup>3</sup>](https://storage.examlex.com/TB1819/11ead62c_1f6c_0796_ac95_8f4117d03f28_TB1819_00.jpg)
The average rate of appearance of B between 20 s and 30 s is mol/s.
A) +5.0 × 10- 4
B) +1.5 × 10- 3
C) - 1.5 × 10- 3
D) - 7.3 × 10- 3
E) +7.3 × 10- 3
![<strong>A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g). The following data are obtained for [A] as the reaction proceeds: The average rate of appearance of B between 20 s and 30 s is mol/s.</strong> A) +5.0 × 10<sup>-</sup><sup> </sup><sup>4</sup> B) +1.5 × 10<sup>-</sup><sup> </sup><sup>3</sup> C) - 1.5 × 10<sup>-</sup><sup> </sup><sup>3</sup> D) - 7.3 × 10<sup>-</sup><sup> </sup><sup>3</sup> E) +7.3 × 10<sup>-</sup><sup> </sup><sup>3</sup>](https://storage.examlex.com/TB1819/11ead62c_1f6c_0796_ac95_8f4117d03f28_TB1819_00.jpg)
The average rate of appearance of B between 20 s and 30 s is mol/s.
A) +5.0 × 10- 4
B) +1.5 × 10- 3
C) - 1.5 × 10- 3
D) - 7.3 × 10- 3
E) +7.3 × 10- 3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
73
A reaction was found to be second order in carbon monoxide concentration. The rate of the reaction If the [CO] is doubled, with everything else kept the same.
A) doubles
B) remains unchanged
C) is reduced by a factor of 2.
D) increases by a factor of 4
E) triples
A) doubles
B) remains unchanged
C) is reduced by a factor of 2.
D) increases by a factor of 4
E) triples

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
74
The rate constant for a second- order reaction is 0.13 M- 1s- 1. If the initial concentration of reactant is 0.26 mol/L, it takes s for the concentration to decrease to 0.13 mol/L.
A) 1.0
B) 0.017
C) 30
D) 4.4 × 10- 3
E) 0.50
A) 1.0
B) 0.017
C) 30
D) 4.4 × 10- 3
E) 0.50

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
75
The graph shown below depicts the relationship between concentration and time for the following chemical reaction.
![<strong>The graph shown below depicts the relationship between concentration and time for the following chemical reaction. The slope of this line is equal to .</strong> A) ln[A]<sub>o</sub><sub> </sub> B) 1/k C) - k D) - 1/k E) k](https://storage.examlex.com/TB1819/11ead62c_1f6c_7cc7_ac95_cb7f9ec7b75a_TB1819_00.jpg)
The slope of this line is equal to .
A) ln[A]o
B) 1/k
C) - k
D) - 1/k
E) k
![<strong>The graph shown below depicts the relationship between concentration and time for the following chemical reaction. The slope of this line is equal to .</strong> A) ln[A]<sub>o</sub><sub> </sub> B) 1/k C) - k D) - 1/k E) k](https://storage.examlex.com/TB1819/11ead62c_1f6c_7cc7_ac95_cb7f9ec7b75a_TB1819_00.jpg)
The slope of this line is equal to .
A) ln[A]o
B) 1/k
C) - k
D) - 1/k
E) k

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
76
The data in the table below were obtained for the reaction:
A + B → P
The order of the reaction in A is .
A) 1
B) 2
C) 3
D) 4
E) 0
A + B → P

The order of the reaction in A is .
A) 1
B) 2
C) 3
D) 4
E) 0

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
77
Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction:
2NO2 → 2NO + O2
In a particular experiment at 300°C, [NO2] drops from 0.0100 to 0.00650 M in 100 s. The rate of appearance of O2 for this period is _ M/s.
A) 7.0 × 10- 5
B) 3.5 × 10- 3
C) 7.0 × 10- 3
D) 3.5 × 10- 5
E) 1.8 × 10- 5
2NO2 → 2NO + O2
In a particular experiment at 300°C, [NO2] drops from 0.0100 to 0.00650 M in 100 s. The rate of appearance of O2 for this period is _ M/s.
A) 7.0 × 10- 5
B) 3.5 × 10- 3
C) 7.0 × 10- 3
D) 3.5 × 10- 5
E) 1.8 × 10- 5

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
78
The average rate disappearance of A between 20 s and 30 s is mol/s.
A) 5.0 × 10- 4
B) 0.15
C) 1.6 × 10- 2
D) 670
E) 1.5 × 10- 3
A) 5.0 × 10- 4
B) 0.15
C) 1.6 × 10- 2
D) 670
E) 1.5 × 10- 3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
79
Reaction rates are affected by reactant concentrations and temperature. This is accounted for by the .

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
80
The overall order of a reaction is 2. The units of the rate constant for the reaction are .
A) M/s
B) 1/M
C) 1/s
D) M- 1s- 1
E) s/M2
A) M/s
B) 1/M
C) 1/s
D) M- 1s- 1
E) s/M2

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck


