Exam 14: Chemical Kinetics
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
The decomposition of N2O5 in solution in carbon tetrachloride proceeds via the reaction 2N2O5 (soln) → 4NO2 (soln) + O2 (soln)
The reaction is first order and has a rate constant of 4.82 × 10- 3 s- 1 at 64°C. If the reaction is initiated with 0.058 mol in a 1.00- L vessel, how many moles remain after 151 s?
Free
(Multiple Choice)
4.9/5  (38)
(38)
Correct Answer:
C
In the Arrhenius equation, k = Ae- Ea/RT Is the frequency factor.
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
B
A reaction was found to be third order in A. Increasing the concentration of A by a factor of 3 will cause the reaction rate to .
Free
(Multiple Choice)
4.7/5  (30)
(30)
Correct Answer:
D
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g). The following data are obtained for [A] as the reaction proceeds: ![A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g). The following data are obtained for [A] as the reaction proceeds: -The average rate of disappearance of A between 10 s and 20 s is mol/s.](https://storage.examlex.com/TB1819/11ead62c_1f6a_a804_ac95_31c6da222cb4_TB1819_00.jpg) -The average rate of disappearance of A between 10 s and 20 s is mol/s.
-The average rate of disappearance of A between 10 s and 20 s is mol/s.
(Multiple Choice)
4.7/5  (35)
(35)
A reaction was found to be second order in carbon monoxide concentration. The rate of the reaction If the [CO] is doubled, with everything else kept the same.
(Multiple Choice)
4.9/5  (35)
(35)
The data in the table below were obtained for the reaction:
A + B → P  -The order of the reaction in A is .
-The order of the reaction in A is .
(Multiple Choice)
4.7/5  (41)
(41)
Which energy difference in the energy profile below corresponds to the activation energy for the forward reaction?
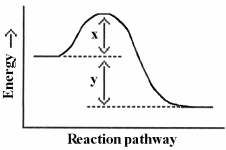
(Multiple Choice)
4.7/5  (33)
(33)
The reaction below is first order in [H2O2]:
2H2O2 (l) - 2H2O (l) + O2 (g)
A solution originally at 0.600 M H2O2 is found to be 0.075 M after 54 min. The half- life for this reaction is min.
(Multiple Choice)
4.9/5  (27)
(27)
For the elementary reaction 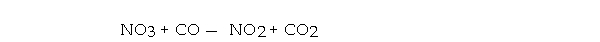 the molecularity of the reaction is , and the rate law is rate = .
the molecularity of the reaction is , and the rate law is rate = .
(Multiple Choice)
4.8/5  (36)
(36)
The isomerization of methylisonitrile to acetonitrile CH3NC (g) → CH3CN (g)
Is first order in CH3NC. The rate constant for the reaction is 9.45 × 10- 5 s- 1 at 478 K. The half- life of the reaction when the initial [CH3NC] is 0.030 M is s.
(Multiple Choice)
4.9/5  (34)
(34)
A particular first- order reaction has a rate constant of 1.35 × 102 s- 1 at 25°C. What is the magnitude of k at 95°C if Ea = 55.5 kJ/mol?
(Multiple Choice)
4.7/5  (35)
(35)
The following reaction is second order in [A] and the rate constant is 0.039 M- 1s- 1:
A - B
The concentration of A was 0.30 M at 23s. The initial concentration of A was _ M.
(Multiple Choice)
4.8/5  (39)
(39)
The data in the table below were obtained for the reaction:
A + B → P  -The overall order of the reaction is .
-The overall order of the reaction is .
(Multiple Choice)
4.7/5  (37)
(37)
The rate limiting step in a reaction is the slowest step in the reaction sequence.
(True/False)
4.9/5  (37)
(37)
If a rate law is second order (reactant) , doubling the reactant _ _ the reaction rate.
(Short Answer)
4.7/5  (44)
(44)
The rate constant of a first- order process that has a half- life of 225 s is s- 1.
(Multiple Choice)
4.8/5  (31)
(31)
The mechanism for formation of the product X is:
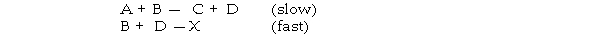 The intermediate reactant in the reaction is .
The intermediate reactant in the reaction is .
(Multiple Choice)
4.9/5  (29)
(29)
The overall reaction order is the sum of the orders of each reactant in the rate law.
(True/False)
4.8/5  (28)
(28)
The half- life of a first- order reaction is 13 min. If the initial concentration of reactant is 0.085 M, it takes min for it to decrease to 0.055 M.
(Multiple Choice)
4.7/5  (33)
(33)
Showing 1 - 20 of 110
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)