Deck 8: Basic Concepts of Chemical Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/116
Play
Full screen (f)
Deck 8: Basic Concepts of Chemical Bonding
1
For the questions that follow, consider the BEST Lewis structures of the following ox_yanions: 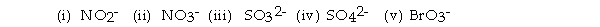
There can be four equivalent best resonance structures of .
A) (i)
B) (ii)
C) (iii)
D) (iv)
E) (v)
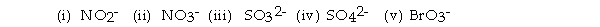
There can be four equivalent best resonance structures of .
A) (i)
B) (ii)
C) (iii)
D) (iv)
E) (v)
(iv)
2
The diagram below is the Born- huber cycle for the formation of crystalline potassium fluoride. 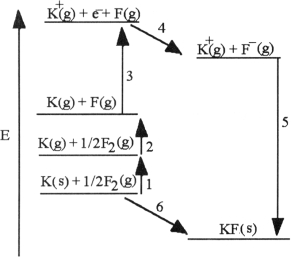
Which energy change corresponds to the first ionization energy of potassium?
A) 6
B) 3
C) 2
D) 4
E) 5

Which energy change corresponds to the first ionization energy of potassium?
A) 6
B) 3
C) 2
D) 4
E) 5
3
3
Which ion below has a noble gas electron configuration?
A) Be2+
B) N2-
C) C2+
D) B2+
E) Li2+
A) Be2+
B) N2-
C) C2+
D) B2+
E) Li2+
Be2+
4
Which two bonds are most similar in polarity?
A) B-F and Cl-F
B) O-F and Cl-F
C) Al-Cl and I-Br
D) Cl-Cl and Be-Cl
E) I-Br and Si-Cl
A) B-F and Cl-F
B) O-F and Cl-F
C) Al-Cl and I-Br
D) Cl-Cl and Be-Cl
E) I-Br and Si-Cl

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
5
Which atom can accommodate an octet of electrons, but doesn't necessarily have to accommodate an octet?
A) B
B) O
C) C
D) H
E) N
A) B
B) O
C) C
D) H
E) N

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following does not have eight valence electrons?
A) Xe
B) Rb+
C) Ca+
D) Br-
E) All of the above have eight valence electrons.
A) Xe
B) Rb+
C) Ca+
D) Br-
E) All of the above have eight valence electrons.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
7
A valid Lewis structure of _ _ cannot be drawn without violating the octet rule.
A) CF4
B) SO32-
C) NF3
D) SO2
E) BeH2
A) CF4
B) SO32-
C) NF3
D) SO2
E) BeH2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
8
Of the atoms below, is the least electronegative.
A) Rb
B) F
C) Ca
D) Cl
E) Si
A) Rb
B) F
C) Ca
D) Cl
E) Si

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
9
In the nitrite ion (NO2- ), .
A) one bond is a double bond and the other is a single bond
B) both bonds are single bonds
C) both bonds are the same
D) both bonds are double bonds
E) there are 20 valence electrons
A) one bond is a double bond and the other is a single bond
B) both bonds are single bonds
C) both bonds are the same
D) both bonds are double bonds
E) there are 20 valence electrons

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
10
Of the atoms below, is the most electronegative.
A) Rb
B) Cl
C) Si
D) Ca
E) S
A) Rb
B) Cl
C) Si
D) Ca
E) S

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
11
Lattice energy is .
A) the energy required to produce one mole of an ionic compound from its constituent elements in their standard states
B) the energy required to convert a mole of ionic solid into its constituent ions in the gas phase
C) the sum of electron affinities of the components in an ionic solid
D) the energy given off when gaseous ions combine to form one mole of an ionic solid
E) the sum of ionization energies of the components in an ionic solid
A) the energy required to produce one mole of an ionic compound from its constituent elements in their standard states
B) the energy required to convert a mole of ionic solid into its constituent ions in the gas phase
C) the sum of electron affinities of the components in an ionic solid
D) the energy given off when gaseous ions combine to form one mole of an ionic solid
E) the sum of ionization energies of the components in an ionic solid

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
12
The Lewis structure of N2H2 shows .
A) each hydrogen has one nonbonding electron pair
B) a nitrogen- nitrogen single bond
C) each nitrogen has two nonbinding electron pairs
D) a nitrogen- nitrogen triple bond
E) each nitrogen has one nonbinding electron pair
A) each hydrogen has one nonbonding electron pair
B) a nitrogen- nitrogen single bond
C) each nitrogen has two nonbinding electron pairs
D) a nitrogen- nitrogen triple bond
E) each nitrogen has one nonbinding electron pair

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
13
Given that the average bond energies for C- H and C- Br bonds are 413 and 276 kJ/mol, respectively, the heat of atomization of bromoform (CHBr3) is _ kJ/mol.
A) 1241
B) 1378
C) - 689
D) 689
E) - 1378
A) 1241
B) 1378
C) - 689
D) 689
E) - 1378

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
14
Of the ions below, only has a noble gas electron configuration.
A) S3-
B) Cl-
C) I+
D) O2+
E) K-
A) S3-
B) Cl-
C) I+
D) O2+
E) K-

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
15
To convert from one resonance structure to another, .
A) neither electrons nor atoms can be moved
B) electrons and atoms can both be moved
C) only atoms can be moved
D) electrons must be added
E) only electrons can be moved
A) neither electrons nor atoms can be moved
B) electrons and atoms can both be moved
C) only atoms can be moved
D) electrons must be added
E) only electrons can be moved

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
16
For the questions that follow, consider the BEST Lewis structures of the following ox_yanions:
(i) NO2-
(ii) NO3-
(iii) SO32-
(iv) SO42-
(v) BrO3-
In which of the ions do all X- O bonds (X indicates the central atom) have the same length?
A) none
B) all
C) (i) and (ii)
D) (iii) and (v)
E) (iii), (iv), and (v)
(i) NO2-
(ii) NO3-
(iii) SO32-
(iv) SO42-
(v) BrO3-
In which of the ions do all X- O bonds (X indicates the central atom) have the same length?
A) none
B) all
C) (i) and (ii)
D) (iii) and (v)
E) (iii), (iv), and (v)

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
17
Of the atoms below, is the most electronegative.
A) O
B) Cl
C) Br
D) F
E) N
A) O
B) Cl
C) Br
D) F
E) N

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
18
The chloride of which of the following metals should have the greatest lattice energy?
A) potassium
B) lithium
C) rubidium
D) sodium
E) cesium
A) potassium
B) lithium
C) rubidium
D) sodium
E) cesium

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
19
Of the following, cannot accommodate more than an octet of electrons.
A) O
B) As
C) S
D) I
E) P
A) O
B) As
C) S
D) I
E) P

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
20
Resonance structures differ by .
A) placement of atoms only
B) placement of electrons only
C) number of electrons only
D) number and placement of electrons
E) number of atoms only
A) placement of atoms only
B) placement of electrons only
C) number of electrons only
D) number and placement of electrons
E) number of atoms only

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
21
A valid Lewis structure of _ _ cannot be drawn without violating the octet rule.
A) NF3
B) SeF4
C) SiF4
D) PO43-
E) CF4
A) NF3
B) SeF4
C) SiF4
D) PO43-
E) CF4

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
22
The type of compound that is most likely to contain a covalent bond is _.
A) one that is composed of only nonmetals
B) one that is composed of a metal from the far left of the periodic table and a nonmetal from the far right of the periodic table
C) held together by the electrostatic forces between oppositely charged ions
D) a solid metal
E) There is no general rule to predict covalency in bonds.
A) one that is composed of only nonmetals
B) one that is composed of a metal from the far left of the periodic table and a nonmetal from the far right of the periodic table
C) held together by the electrostatic forces between oppositely charged ions
D) a solid metal
E) There is no general rule to predict covalency in bonds.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
23
A valid Lewis structure of _ _ cannot be drawn without violating the octet rule.
A) ICl5
B) SiF4
C) CO2
D) SO2
E) NI3
A) ICl5
B) SiF4
C) CO2
D) SO2
E) NI3

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
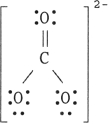
24
The Lewis structure of the CO32- ion is .
A)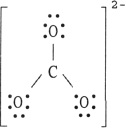
B)
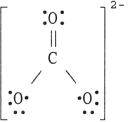
C)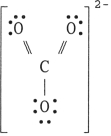
D)
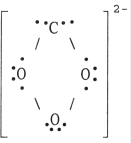
E)
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
25
Using the Born- Haber cycle, the OHf° of KBr is equal to _ _.
A) OHf°[K (g)] + OHf°[Br (g)] - I1 - E(Br) + OHlattice
B) OHf°[K (g)] + OHf°[Br (g)] + I1(K) + E(Br) + OHlattice
C) OHf°[K (g)] - OHf°[Br (g)] + I1(K) - E(Br) + OHlattice
D) OHf°[K (g)] + OHf°[Br (g)] + I1(K) + E(Br) - OHlattice
E) OHf°[K (g)] - OHf°[Br (g)] - I1(K) - E(Br) - OHlattice
A) OHf°[K (g)] + OHf°[Br (g)] - I1 - E(Br) + OHlattice
B) OHf°[K (g)] + OHf°[Br (g)] + I1(K) + E(Br) + OHlattice
C) OHf°[K (g)] - OHf°[Br (g)] + I1(K) - E(Br) + OHlattice
D) OHf°[K (g)] + OHf°[Br (g)] + I1(K) + E(Br) - OHlattice
E) OHf°[K (g)] - OHf°[Br (g)] - I1(K) - E(Br) - OHlattice

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
26
Of the possible bonds between carbon atoms (single, double, and triple), _.
A) a double bond is longer than a triple bond
B) a single bond is stronger than a double bond
C) a triple bond is longer than a single bond
D) a double bond is stronger than a triple bond
E) a single bond is stronger than a triple bond
A) a double bond is longer than a triple bond
B) a single bond is stronger than a double bond
C) a triple bond is longer than a single bond
D) a double bond is stronger than a triple bond
E) a single bond is stronger than a triple bond

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
27
For resonance forms of a molecule or ion, .
A) there cannot be more than two resonance structures for a given species
B) the observed structure is an average of the resonance forms
C) all the resonance structures are observed in various proportions
D) the same atoms need not be bonded to each other in all resonance forms
E) one always corresponds to the observed structure
A) there cannot be more than two resonance structures for a given species
B) the observed structure is an average of the resonance forms
C) all the resonance structures are observed in various proportions
D) the same atoms need not be bonded to each other in all resonance forms
E) one always corresponds to the observed structure

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following names is/are correct for the compound SnCl4?
A) tin chloride and tin (II) tetrachloride
B) tin (IV) tetrachloride
C) tin tetrachloride and tin (IV) chloride
D) tin (II) chloride and tin (IV) chloride
E) tin chloride
A) tin chloride and tin (II) tetrachloride
B) tin (IV) tetrachloride
C) tin tetrachloride and tin (IV) chloride
D) tin (II) chloride and tin (IV) chloride
E) tin chloride

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
29
Dynamite .
A) is made of nitroglycerine and an absorbent such as diatomaceous earth
B) was invented by Alfred Nobel
C) is a much safer explosive than pure nitroglycerine
D) is an explosive
E) all of the above
A) is made of nitroglycerine and an absorbent such as diatomaceous earth
B) was invented by Alfred Nobel
C) is a much safer explosive than pure nitroglycerine
D) is an explosive
E) all of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
30
Bond enthalpy is .
A) always zero
B) always positive
C) sometimes positive, sometimes negative
D) always negative
E) unpredictable
A) always zero
B) always positive
C) sometimes positive, sometimes negative
D) always negative
E) unpredictable

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
31
The bond length in an HI molecule is 1.61 Å and the measured dipole moment is 0.44 D. What is the magnitude (in units of e) of the negative charge on I in HI?
(1 debye = 3.34 × 10- 34 coulomb- meters; ; e=1.6 × 10- 19 coulombs)
A) 1.6 × 10- 19
B) 1
C) 0.057
D) 0.22
E) 9.1
(1 debye = 3.34 × 10- 34 coulomb- meters; ; e=1.6 × 10- 19 coulombs)
A) 1.6 × 10- 19
B) 1
C) 0.057
D) 0.22
E) 9.1

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
32
Of the bonds below, is the least polar.
A) Na, S
B) P, S
C) Si, Cl
D) C, F
E) Na, Cl
A) Na, S
B) P, S
C) Si, Cl
D) C, F
E) Na, Cl

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
33
A valid Lewis structure of _ _ cannot be drawn without violating the octet rule.
A) ClF3
B) SO3
C) CCl4
D) CO2
E) PCl3
A) ClF3
B) SO3
C) CCl4
D) CO2
E) PCl3

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
34
The central atom in _ _ does not violate the octet rule.
A) SF4
B) CF4
C) KrF2
D) XeF4
E) ICl4-
A) SF4
B) CF4
C) KrF2
D) XeF4
E) ICl4-

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following has the bonds correctly arranged in order of increasing polarity?
A) N-F, Be-F, Mg-F, O-F
B) Be-F, Mg-F, N-F, O-F
C) O-F, N-F, Be-F, Mg-F
D) Mg-F, Be-F, N-F, O-F
E) O-F, Be-F, Mg-F, N-F
A) N-F, Be-F, Mg-F, O-F
B) Be-F, Mg-F, N-F, O-F
C) O-F, N-F, Be-F, Mg-F
D) Mg-F, Be-F, N-F, O-F
E) O-F, Be-F, Mg-F, N-F

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
36
The central atom in _ violates the octet rule.
A) NH3
B) AsF3
C) BF3
D) SeF2
E) CF4
A) NH3
B) AsF3
C) BF3
D) SeF2
E) CF4

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
37
In which of the molecules below is the carbon- carbon distance the shortest?
A) H3C- CH3
B) H3C- CH2- CH3
C) H- C≡C- H
D) H2C=CH2
E) H2C=C=CH2
A) H3C- CH3
B) H3C- CH2- CH3
C) H- C≡C- H
D) H2C=CH2
E) H2C=C=CH2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
38
Of the molecules below, the bond in is the most polar.
A) HF
B) HI
C) H2
D) HCl
E) HBr
A) HF
B) HI
C) H2
D) HCl
E) HBr

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
39
A valid Lewis structure of _ _ cannot be drawn without violating the octet rule.
A) SbF3
B) SO42-
C) NF3
D) PF3
E) IF3
A) SbF3
B) SO42-
C) NF3
D) PF3
E) IF3

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following has eight valence electrons?
A) Cl-
B) Kr
C) Ti4+
D) Na+
E) all of the above
A) Cl-
B) Kr
C) Ti4+
D) Na+
E) all of the above

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
41
The electron configuration that corresponds to the Lewis symbol, :

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following names is/are correct for the compound TiO2?
A) titanium oxide
B) titanium (IV) dioxide
C) titanium dioxide and titanium (IV) oxide
D) titanium (II) oxide
E) titanium oxide and titanium (IV) dioxide
A) titanium oxide
B) titanium (IV) dioxide
C) titanium dioxide and titanium (IV) oxide
D) titanium (II) oxide
E) titanium oxide and titanium (IV) dioxide

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
43
The electron configuration of the S2- ion is .
A) [Ne]3s23p6
B) [Ar]3s23p2
C) [Ar]3s23p6
D) [Ne]3s23p2
E) [Kr]3s22p- 6
A) [Ne]3s23p6
B) [Ar]3s23p2
C) [Ar]3s23p6
D) [Ne]3s23p2
E) [Kr]3s22p- 6

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
44
The diagram below is the Born- huber cycle for the formation of crystalline potassium fluoride.
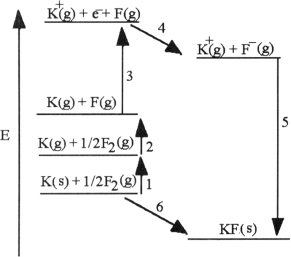
Which energy change corresponds to the electron affinity of fluorine?
A) 1
B) 5
C) 4
D) 2
E) 6

Which energy change corresponds to the electron affinity of fluorine?
A) 1
B) 5
C) 4
D) 2
E) 6

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
45
Alternative but equivalent Lewis structures are called _ _ .

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
46
In a reaction, if the bonds in the reactants are stronger than the bonds in the product, the reaction is .

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
47
Which halogen, bromine or iodine, will form the more polar bond with phophorus ?

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the elements below has the largest electronegativity?
A) P
B) Mg
C) Na
D) Si
E) S
A) P
B) Mg
C) Na
D) Si
E) S

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
49
Of the bonds C- N, C=N, and C÷N, the C- N bond is .
A) strongest/shortest
B) weakest/longest
C) weakest/shortest
D) strongest/longest
E) intermediate in both strength and length
A) strongest/shortest
B) weakest/longest
C) weakest/shortest
D) strongest/longest
E) intermediate in both strength and length

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
50
As the number of covalent bonds between two atoms increases, the distance between the atoms
And the strength of the bond between them _ _.
A) increases, increases
B) decreases, decreases
C) increases, decreases
D) decreases, increases
E) is unpredictable
And the strength of the bond between them _ _.
A) increases, increases
B) decreases, decreases
C) increases, decreases
D) decreases, increases
E) is unpredictable

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
51
The strength of a covalent bond is measured by its .

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
52
Dynamite consists of nitroglycerine mixed with .
A) diatomaceous earth or cellulose
B) potassium nitrate
C) damp KOH
D) TNT
E) solid carbon
A) diatomaceous earth or cellulose
B) potassium nitrate
C) damp KOH
D) TNT
E) solid carbon

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
53
The principal quantum number of the electrons that are lost when tungsten forms a caton is
A) 6
B) 5
C) 4
D) 3
E) 2
A) 6
B) 5
C) 4
D) 3
E) 2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
54
Calculate the bond energy of C- F given that the heat of atomization of CHFClBr is 1502 kJ/mol, and that the bond energies of C- H, C- Br, and C- Cl are 413, 276, and 328 kJ/mol, respectively.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
55
Draw the Lewis structure of ICl2+.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
56
From the information given below, calculate the heat of combustion of methane (CH4) (in kJ/mol). Start by writing the balanced equation. 


Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
57
Most explosives are compounds that decompose rapidly to produce products and a great deal of .
A) solid, gas
B) liquid, heat
C) gaseous, gases
D) gaseous, heat
E) soluble, heat
A) solid, gas
B) liquid, heat
C) gaseous, gases
D) gaseous, heat
E) soluble, heat

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
58
Write the balanced chemical equation for the reaction for which OH°rxn is the lattice energy for potassium bromide.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
59
Why don't we draw double bonds between the Be atom and the Cl atoms in BeCl2?
A) That would give positive formal charges to the chlorine atoms and a negative formal charge to the beryllium atom.
B) That would result in more than eight electrons around each chlorine atom.
C) That would result in the formal charges not adding up to zero.
D) There aren't enough electrons.
E) That would result in more than eight electrons around beryllium.
A) That would give positive formal charges to the chlorine atoms and a negative formal charge to the beryllium atom.
B) That would result in more than eight electrons around each chlorine atom.
C) That would result in the formal charges not adding up to zero.
D) There aren't enough electrons.
E) That would result in more than eight electrons around beryllium.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
60
Give the electron configuration of Cu2+.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
61
For a given arrangement of ions, the lattice energy increases as ionic radius and as ionic charge .
A) decreases, increases
B) decreases, decreases
C) increases, decreases
D) increases, increases
E) This cannot be predicted.
A) decreases, increases
B) decreases, decreases
C) increases, decreases
D) increases, increases
E) This cannot be predicted.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
62
How many unpaired electrons are there in the Lewis structures of a N3- ion?
A) 0
B) 1
C) 2
D) 3
E) This cannot be predicted.
A) 0
B) 1
C) 2
D) 3
E) This cannot be predicted.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
63
Electronegativity _ from left to right within a period and _ from top to bottom within a group.
A) increases, increases
B) stays the same, increases
C) decreases, increases
D) increases, stays the same
E) increases, decreases
A) increases, increases
B) stays the same, increases
C) decreases, increases
D) increases, stays the same
E) increases, decreases

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
64
The formal charge on sulfur in SO42- is , where the Lewis structure of the ion is:
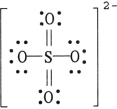
A) 0
B) - 4
C) - 2
D) +4
E) +2

A) 0
B) - 4
C) - 2
D) +4
E) +2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
65
Using the table of bond dissociation energies, the OH for the following reaction is kJ. 

A) - 359
B) - 223
C) 359
D) 208
E) 223


A) - 359
B) - 223
C) 359
D) 208
E) 223

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following would have to lose two electrons in order to achieve a noble gas electron configuration ? 
A) O, Se
B) Sr, O, Se
C) Sr
D) Na
E) Br

A) O, Se
B) Sr, O, Se
C) Sr
D) Na
E) Br

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
67
What is the maximum number of double bonds that a carbon atom can form?
A) 0
B) 3
C) 4
D) 2
E) 1
A) 0
B) 3
C) 4
D) 2
E) 1

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
68
A nonpolar bond will form between two _ _ atoms of electronegativity.
A) identical, equal
B) similar, different
C) different, opposite
D) different, different
E) identical, different
A) identical, equal
B) similar, different
C) different, opposite
D) different, different
E) identical, different

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
69
Using the table of bond dissociation energies, the OH for the following gas- phase reaction is
KJ)
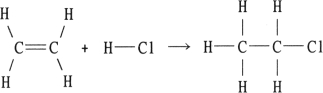
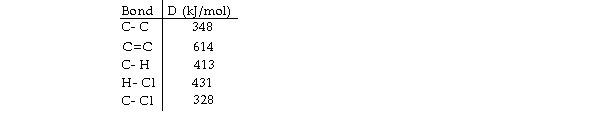
A) - 44
B) - 38
C) 304
D) 38
E) 2134
KJ)
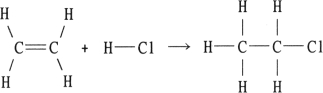
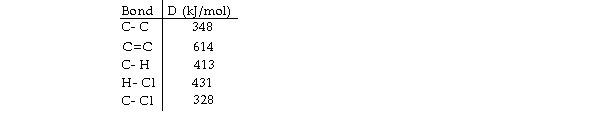
A) - 44
B) - 38
C) 304
D) 38
E) 2134

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
70
Based on the octet rule, iodine most likely forms an _ ion.
A) I4-
B) I2+
C) I+
D) I4+
E) I-
A) I4-
B) I2+
C) I+
D) I4+
E) I-

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following would have to gain two electrons in order to achieve a noble gas electron configuration _ ?
O Sr Na Se Br
A) Br
B) Sr, O, Se
C) Sr
D) O, Se
E) Na
O Sr Na Se Br
A) Br
B) Sr, O, Se
C) Sr
D) O, Se
E) Na

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
72
The ion ICI4- has _ valence electrons.
A) 34
B) 8
C) 35
D) 36
E) 28
A) 34
B) 8
C) 35
D) 36
E) 28

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
73
Determining lattice energy from Born- Haber cycle data requires the use of .
A) Avogadro's number
B) the octet rule
C) Coulomb's law
D) Hess's law
E) Periodic law
A) Avogadro's number
B) the octet rule
C) Coulomb's law
D) Hess's law
E) Periodic law

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
74
How many single covalent bonds must a silicon atom form to have a complete octet in its valence shell?
A) 3
B) 4
C) 2
D) 0
E) 1
A) 3
B) 4
C) 2
D) 0
E) 1

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
75
The formal charge on carbon in the molecule below is .
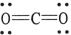
A) +1
B) +2
C) +3
D) - 1
E) 0
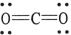
A) +1
B) +2
C) +3
D) - 1
E) 0

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
76
In the Lewis symbol for a fluorine atom, there are paired and unpaired electrons.
A) 4,1
B) 4, 2
C) 2, 5
D) 0, 5
E) 6, 1
A) 4,1
B) 4, 2
C) 2, 5
D) 0, 5
E) 6, 1

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
77
How many unpaired electrons are there in an O2- ion?
A) 0
B) 1
C) 2
D) 3
E) This cannot be predicted.
A) 0
B) 1
C) 2
D) 3
E) This cannot be predicted.

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
78
A covalent bond between the same two atoms is the longest.
A) triple
B) single
C) double
D) They are all the same length.
E) strong
A) triple
B) single
C) double
D) They are all the same length.
E) strong

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
79
What is the electron configuration for the Co2+ ion?
A) [Ar]4s23d9
B) [Ar]4s03d7
C) [Ar]4s13d6
D) [Ne]3s23p10
E) [Ar]4s03d5
A) [Ar]4s23d9
B) [Ar]4s03d7
C) [Ar]4s13d6
D) [Ne]3s23p10
E) [Ar]4s03d5

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck
80
How many equivalent resonance forms can be drawn for CO32- - (carbon is the central atom)?
A) 1
B) 0
C) 3
D) 4
E) 2
A) 1
B) 0
C) 3
D) 4
E) 2

Unlock Deck
Unlock for access to all 116 flashcards in this deck.
Unlock Deck
k this deck


