Exam 8: Basic Concepts of Chemical Bonding
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
In the Lewis structure of ClF, the formal charge on Cl is _ and the formal charge on F is
Free
(Multiple Choice)
4.7/5  (34)
(34)
Correct Answer:
E
The Lewis structure of HCN (H bonded to C) shows that has nonbonding electron pairs.
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
E
Elements from opposite sides of the periodic table tend to form .
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
E
Determining lattice energy from Born- Haber cycle data requires the use of .
(Multiple Choice)
4.9/5  (32)
(32)
A valid Lewis structure of _ _ cannot be drawn without violating the octet rule.
(Multiple Choice)
4.8/5  (35)
(35)
The Lewis structure of AsH3 shows nonbonding electron pair(s) on As.
(Multiple Choice)
4.8/5  (38)
(38)
The diagram below is the Born- huber cycle for the formation of crystalline potassium fluoride. 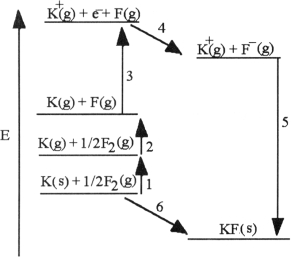 -Which energy change corresponds to the first ionization energy of potassium?
-Which energy change corresponds to the first ionization energy of potassium?
(Multiple Choice)
4.7/5  (23)
(23)
How many hydrogen atoms must bond to silicon to give it an octet of valence electrons?
(Multiple Choice)
4.8/5  (41)
(41)
The halogens, alkali metals, and alkaline earth metals have valence electrons, respectively.
(Multiple Choice)
4.8/5  (34)
(34)
The Lewis structure of PF3 shows that the central phosphorus atom has nonbonding and bonding electron pairs.
(Multiple Choice)
4.9/5  (40)
(40)
Based on the octet rule, iodine most likely forms an _ ion.
(Multiple Choice)
4.8/5  (32)
(32)
Showing 1 - 20 of 116
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)