Deck 3: Stoichiometry: Calculations With Chemical Formulas and Equations
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/134
Play
Full screen (f)
Deck 3: Stoichiometry: Calculations With Chemical Formulas and Equations
1
The mass % of H in methane (CH4) is .
A) 74.87
B) 4.032
C) 25.13
D) 92.26
E) 7.743
A) 74.87
B) 4.032
C) 25.13
D) 92.26
E) 7.743
25.13
2
The formula weight of ammonium sulfate ((NH4)2SO4) is _ amu.
A) 264
B) 100
C) 132
D) 116
E) 118
A) 264
B) 100
C) 132
D) 116
E) 118
132
3
Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements:
 How many grams of sodium azide are required to produce 18.0 g of nitrogen?
How many grams of sodium azide are required to produce 18.0 g of nitrogen?
A) 62.7
B) 0.964
C) 0.428
D) 41.8
E) 27.9
 How many grams of sodium azide are required to produce 18.0 g of nitrogen?
How many grams of sodium azide are required to produce 18.0 g of nitrogen?A) 62.7
B) 0.964
C) 0.428
D) 41.8
E) 27.9
27.9
4
Lithium and nitrogen react in a combination reaction to produce lithium nitride:

How many moles of N2 are needed to react with 0.500 mol of lithium?
A) 1.50
B) 0.0833
C) 3.00
D) 0.167
E) 0.500

How many moles of N2 are needed to react with 0.500 mol of lithium?
A) 1.50
B) 0.0833
C) 3.00
D) 0.167
E) 0.500

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
5
The mass % of Al in aluminum sulfate (Al2(SO4)3) is .
A) 45.70
B) 21.93
C) 35.94
D) 7.886
E) 15.77
A) 45.70
B) 21.93
C) 35.94
D) 7.886
E) 15.77

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
6
The formula weight of aluminum sulfate (Al2(SO4)3) is _ amu.
A) 123.04
B) 342.14
C) 273.06
D) 59.04
E) 150.14
A) 123.04
B) 342.14
C) 273.06
D) 59.04
E) 150.14

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
7
How many oxygen atoms are contained in 2.74 g of Al2(SO4)3?
A) 12
B) 6.02 × 1023
C) 5.79 × 1022
D) 8.01 × 10- 3
E) 7.22 × 1024
A) 12
B) 6.02 × 1023
C) 5.79 × 1022
D) 8.01 × 10- 3
E) 7.22 × 1024

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
8
The formula weight of magnesium fluoride (MgF2) is _ amu.
A) 92.9
B) 62.3
C) 86.6
D) 67.6
E) 43.3
A) 92.9
B) 62.3
C) 86.6
D) 67.6
E) 43.3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
9
How many oxygen atoms are there in 52.06 g of carbon dioxide?
A) 1.018 × 1024
B) 1.424 × 1024
C) 6.022 × 1023
D) 5.088 × 1023
E) 1.204 × 1024
A) 1.018 × 1024
B) 1.424 × 1024
C) 6.022 × 1023
D) 5.088 × 1023
E) 1.204 × 1024

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
10
How many sulfur dioxide molecules are there in 0.180 mol of sulfur dioxide?
A) 1.08 × 1024
B) 6.02 × 1024
C) 6.02 × 1023
D) 1.80 × 1023
E) 1.08 × 1023
A) 1.08 × 1024
B) 6.02 × 1024
C) 6.02 × 1023
D) 1.80 × 1023
E) 1.08 × 1023

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
11
Magnesium burns in air with a dazzling brilliance to produce magnesium oxide:
2Mg (s) + O2 (g) - 2MgO (s)
How many moles of O2 are consumed when 0.770 mol of magnesium burns?
A) 1.54
B) 0.385
C) 2.60
D) 0.770
E) 0.0317
2Mg (s) + O2 (g) - 2MgO (s)
How many moles of O2 are consumed when 0.770 mol of magnesium burns?
A) 1.54
B) 0.385
C) 2.60
D) 0.770
E) 0.0317

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
12
Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements:

How many moles of N2 are produced by the decomposition of 2.88 mol of sodium azide?
A) 4.32
B) 1.92
C) 0.960
D) 1.44
E) 8.64

How many moles of N2 are produced by the decomposition of 2.88 mol of sodium azide?
A) 4.32
B) 1.92
C) 0.960
D) 1.44
E) 8.64

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
13
Calculate the percentage by mass of nitrogen in PtCl2(NH3)2.
A) 4.67
B) 9.34
C) 4.95
D) 12.67
E) 9.90
A) 4.67
B) 9.34
C) 4.95
D) 12.67
E) 9.90

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
14
Lithium and nitrogen react in a combination reaction to produce lithium nitride:

How many moles of lithium are needed to produce 0.60 mol of Li3N when the reaction is carried out in the presence of excess nitrogen?
A) 0.20
B) 3.6
C) 1.8
D) 0.40
E) 0.30

How many moles of lithium are needed to produce 0.60 mol of Li3N when the reaction is carried out in the presence of excess nitrogen?
A) 0.20
B) 3.6
C) 1.8
D) 0.40
E) 0.30

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
15
When a hydrocarbon burns in air, what component of air reacts?
A) nitrogen
B) water
C) oxygen
D) carbon dioxide
E) argon
A) nitrogen
B) water
C) oxygen
D) carbon dioxide
E) argon

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
16
What is the mass % of carbon in dimethylsulfoxide (C2H6SO)?
A) 79.8
B) 60.0
C) 20.6
D) 7.74
E) 30.7
A) 79.8
B) 60.0
C) 20.6
D) 7.74
E) 30.7

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
17
What is the empirical formula of a compound that is 64.8% C, 13.6% H, and 21.6% O by mass?
A) C5HO2
B) C4HO1
C) C8H20O2
D) C5H14O1
E) C4H10O1
A) C5HO2
B) C4HO1
C) C8H20O2
D) C5H14O1
E) C4H10O1

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
18
How many grams of sodium carbonate contain 1.773 × 1017 carbon atoms?
A) 3.121 × 10- 5
B) 1.011 × 10- 5
C) 6.066 × 10- 5
D) 1.517 × 10- 5
E) 9.100 × 10- 5
A) 3.121 × 10- 5
B) 1.011 × 10- 5
C) 6.066 × 10- 5
D) 1.517 × 10- 5
E) 9.100 × 10- 5

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
19
Which hydrocarbon pair below have identical mass percentage of C?
A) C2H4 and C4H2
B) C2H4 and C3H4
C) C3H4 and C3H6
D) C2H4 and C3H6
E) none of the above
A) C2H4 and C4H2
B) C2H4 and C3H4
C) C3H4 and C3H6
D) C2H4 and C3H6
E) none of the above

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
20
The mass % of C in methane (CH4) is .
A) 7.743
B) 74.87
C) 92.26
D) 25.13
E) 133.6
A) 7.743
B) 74.87
C) 92.26
D) 25.13
E) 133.6

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
21
One mole of contains the largest number of atoms.
A) C10H8
B) Na3PO4
C) Cl2
D) S8
E) Al2(SO4)3
A) C10H8
B) Na3PO4
C) Cl2
D) S8
E) Al2(SO4)3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
22
What is the mass in grams of 9.76 × 1012 atoms of naturally occurring sodium?
A) 1.62 × 10- 11
B) 7.05 × 10- 13
C) 2.24 × 1014
D) 22.99
E) 3.73 × 10- 10
A) 1.62 × 10- 11
B) 7.05 × 10- 13
C) 2.24 × 1014
D) 22.99
E) 3.73 × 10- 10

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
23
Calculate the percentage by mass of chlorine in PtCl2(NH3)2.
A) 23.63
B) 12.53
C) 25.05
D) 11.82
E) 18.09
A) 23.63
B) 12.53
C) 25.05
D) 11.82
E) 18.09

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
24
The molecular weight of the acetic acid (CH3CO2H) is amu.
A) 60
B) 48
C) 32
D) 44
A) 60
B) 48
C) 32
D) 44

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
25
The formula of nitrobenzene is C6H5NO2. The molecular weight of this compound is amu.
A) 109.10
B) 43.03
C) 123.11
D) 107.11
E) 3.06
A) 109.10
B) 43.03
C) 123.11
D) 107.11
E) 3.06

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
26
There are _ atoms of oxygen are in 300 molecules of CH3CO2H.
A) 600
B) 3.01 × 1024
C) 1.80 × 1026
D) 300
E) 3.61 × 1026
A) 600
B) 3.01 × 1024
C) 1.80 × 1026
D) 300
E) 3.61 × 1026

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
27
A sample of CH2F2 with a mass of 19 g contains _ atoms of F.
A) 38
B) 9.5
C) 2.2 × 1023
D) 4.4 × 1023
E) 3.3 × 1024
A) 38
B) 9.5
C) 2.2 × 1023
D) 4.4 × 1023
E) 3.3 × 1024

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate the percentage by mass of hydrogen in PtCl2(NH3)2.
A) 1.008
B) 0.034
C) 2.016
D) 0.672
E) 1.558
A) 1.008
B) 0.034
C) 2.016
D) 0.672
E) 1.558

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
29
The molecular weight of the ethanol (C2H5OH) is amu.
A) 30
B) 92
C) 34
D) 41
E) 46
A) 30
B) 92
C) 34
D) 41
E) 46

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
30
Lithium and nitrogen react in a combination reaction to produce lithium nitride:
6Li (s) + N2 (g) -2Li3N (s)
How many moles of lithium nitride are produced when 0.450 mol of lithium react in this fashion?
A) 1.35
B) 0.0750
C) 0.150
D) 0.225
E) 0.900
6Li (s) + N2 (g) -2Li3N (s)
How many moles of lithium nitride are produced when 0.450 mol of lithium react in this fashion?
A) 1.35
B) 0.0750
C) 0.150
D) 0.225
E) 0.900

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
31
The formula weight of a substance is .
A) the weight of a sample of the substance
B) identical to the molar mass
C) the same as the percent by mass weight
D) the sum of the atomic weights of each atom in its chemical formula
E) determined by combustion analysis
A) the weight of a sample of the substance
B) identical to the molar mass
C) the same as the percent by mass weight
D) the sum of the atomic weights of each atom in its chemical formula
E) determined by combustion analysis

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
32
How many atoms of nitrogen are in 10 g of NH4NO3?
A) 2
B) 3.0 × 1023
C) 1.8
D) 3.5
E) 1.5 × 1023
A) 2
B) 3.0 × 1023
C) 1.8
D) 3.5
E) 1.5 × 1023

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
33
Gaseous argon has a density of 1.40 g/L at standard conditions. How many argon atoms are in 1.00 L of argon gas at standard conditions?
A) 4.7 × 1022
B) 2.1 × 1022
C) 3.4 × 1025
D) 1.5 × 1025
E) 6.02 × 1023
A) 4.7 × 1022
B) 2.1 × 1022
C) 3.4 × 1025
D) 1.5 × 1025
E) 6.02 × 1023

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
34
A nitrogen oxide is 63.65% by mass nitrogen. The molecular formula could be _ _.
A) N2O4
B) N2O
C) NO2
D) NO
E) either NO2 or N2O4
A) N2O4
B) N2O
C) NO2
D) NO
E) either NO2 or N2O4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
35
A sulfur oxide is 50.0% by mass sulfur. This molecular formula could be .
A) S2O4
B) SO2
C) S2O
D) SO
E) either SO2 or S2O4
A) S2O4
B) SO2
C) S2O
D) SO
E) either SO2 or S2O4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
36
The formula weight of potassium phosphate (K3PO4) is _ amu.
A) 212.27
B) 86.07
C) 251.37
D) 173.17
E) 196.27
A) 212.27
B) 86.07
C) 251.37
D) 173.17
E) 196.27

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
37
How many sulfur dioxide molecules are there in 1.80 mol of sulfur dioxide?
A) 1.80 × 1024
B) 6.02 × 1024
C) 6.02 × 1023
D) 1.08 × 1023
E) 1.08 × 1024
A) 1.80 × 1024
B) 6.02 × 1024
C) 6.02 × 1023
D) 1.08 × 1023
E) 1.08 × 1024

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
38
Of the reactions below, which one is a decomposition reaction?
A) 2CH4 + 4O2 -2CO2 + 4H2O
B) 2Mg + O2 - 2MgO
C) Cd(NO3)2 + Na2S - CdS + 2NaNO3
D) 2N2 + 3H2 - 2NH3
E) NH4Cl - NH3 + HCl
A) 2CH4 + 4O2 -2CO2 + 4H2O
B) 2Mg + O2 - 2MgO
C) Cd(NO3)2 + Na2S - CdS + 2NaNO3
D) 2N2 + 3H2 - 2NH3
E) NH4Cl - NH3 + HCl

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
39
A sample of CH4O with a mass of 32.0 g contains molecules of CH4O.
A) 32.0
B) 1.00
C) 5.32 × 10- 23
D) 1.88 × 1022
E) 6.02 × 1023
A) 32.0
B) 1.00
C) 5.32 × 10- 23
D) 1.88 × 1022
E) 6.02 × 1023

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
40
One million argon atoms is mol of argon atoms.
A) 3
B) 1.0 × 10- 6
C) 1.7 × 10- 18
D) 1.0 × 10+6
E) 6.0 × 1023
A) 3
B) 1.0 × 10- 6
C) 1.7 × 10- 18
D) 1.0 × 10+6
E) 6.0 × 1023

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
41
How many molecules of CH4 are in 48.2 g of this compound?
A) 5.00 × 1024
B) 4.00
C) 3.00
D) 2.90 × 1025
E) 1.81 × 1024
A) 5.00 × 1024
B) 4.00
C) 3.00
D) 2.90 × 1025
E) 1.81 × 1024

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
42
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:

In a particular experiment, a 5.00- g sample of CaO is reacted with excess water and 6.11 g of Ca(OH)2 is recovered. What is the percent yield in this experiment?
A) 7.19
B) 122
C) 81.9
D) 1.22
E) 92.4

In a particular experiment, a 5.00- g sample of CaO is reacted with excess water and 6.11 g of Ca(OH)2 is recovered. What is the percent yield in this experiment?
A) 7.19
B) 122
C) 81.9
D) 1.22
E) 92.4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
43
Of the reactions below, which one is not a combination reaction?
A) 2CH4 + 4O2 -2CO2 + 4H2O
B) CaO + H2O - Ca(OH)2
C) 2Mg + O2 - 2MgO
D) C + O2 - CO2
E) 2N2 + 3H2 - 2NH3
A) 2CH4 + 4O2 -2CO2 + 4H2O
B) CaO + H2O - Ca(OH)2
C) 2Mg + O2 - 2MgO
D) C + O2 - CO2
E) 2N2 + 3H2 - 2NH3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
44
The formula weight of silver chromate (Ag2CrO4) is _ amu.
A) 159.87
B) 175.87
C) 223.87
D) 331.73
E) 339.86
A) 159.87
B) 175.87
C) 223.87
D) 331.73
E) 339.86

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
45
Water can be formed from the stoichiometric reaction of hydrogen with oxygen:

A complete reaction of 5.0 g of O2 with excess hydrogen produces g of H2O.

A complete reaction of 5.0 g of O2 with excess hydrogen produces g of H2O.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
46
How many moles of pyridine (C5H5N) are contained in 3.13 g of pyridine?
A) 0.00404
B) 0.0396
C) 25.3
D) 4.04 × 103
E) 0.319
A) 0.00404
B) 0.0396
C) 25.3
D) 4.04 × 103
E) 0.319

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
47
The reaction used to inflate automobile airbags _.
A) is a decomposition reaction
B) produces sodium gas
C) violates the law of conservation of mass
D) is a combination reaction
E) is a combustion reaction
A) is a decomposition reaction
B) produces sodium gas
C) violates the law of conservation of mass
D) is a combination reaction
E) is a combustion reaction

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
48
Calcium oxide reacts with water in a combination reaction to produce calcium hydroxide:

A 1)50- g sample of CaO is reacted with 1.45 g of H2O. How many grams of water remains after completion of reaction?
A) 0.00
B) 0.0536
C) 0.966
D) 1.04
E) 0.00297

A 1)50- g sample of CaO is reacted with 1.45 g of H2O. How many grams of water remains after completion of reaction?
A) 0.00
B) 0.0536
C) 0.966
D) 1.04
E) 0.00297

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
49
Which one of the following is not true concerning automotive air bags?
A) The gas used for inflating them is oxygen
B) A gas is produced when the air bag activates.
C) They are loaded with sodium azide initially
D) The two products of the decomposition reaction are sodium and nitrogen
E) They are inflated as a result of a decomposition reaction
A) The gas used for inflating them is oxygen
B) A gas is produced when the air bag activates.
C) They are loaded with sodium azide initially
D) The two products of the decomposition reaction are sodium and nitrogen
E) They are inflated as a result of a decomposition reaction

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
50
The formula weight of calcium nitrate (Ca(NO3)2) is _ amu.
A) 150.1
B) 204.2
C) 102.1
D) 116.1
E) 164.0
A) 150.1
B) 204.2
C) 102.1
D) 116.1
E) 164.0

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
51
The formula weight of potassium dichromate (K2Cr2O7) is _ amu.
A) 242.18
B) 107.09
C) 333.08
D) 255.08
E) 294.18
A) 242.18
B) 107.09
C) 333.08
D) 255.08
E) 294.18

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
52
The total number of atoms in 0.111 mol of Fe(CO)3(PH3)2 is _ .
A) 15
B) 1.67
C) 1.07 × 1024
D) 2.76 × 10- 24
E) 4.46 × 1021
A) 15
B) 1.67
C) 1.07 × 1024
D) 2.76 × 10- 24
E) 4.46 × 1021

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
53
Which one of the following substances is the product of this combination reaction?
Al (s) + I2 (s) -
A) AlI
B) AlI3
C) Al2I3
D) Al3I2
E) AlI2
Al (s) + I2 (s) -
A) AlI
B) AlI3
C) Al2I3
D) Al3I2
E) AlI2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
54
The mass % of F in the binary compound KrF2 is _ .
A) 45.38
B) 68.80
C) 18.48
D) 81.52
E) 31.20
A) 45.38
B) 68.80
C) 18.48
D) 81.52
E) 31.20

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
55
The molecular weight of urea ( (NH2)2CO ), a compound used as a nitrogen fertilizer, is
Amu)
A) 43.0
B) 8.0
C) 44.0
D) 32.0
E) 60.1
Amu)
A) 43.0
B) 8.0
C) 44.0
D) 32.0
E) 60.1

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
56
How many carbon atoms are there in 52.06 g of carbon dioxide?
A) 3.134 × 1025
B) 1.424 × 1024
C) 7.122 × 1023
D) 5.206 × 1024
E) 8.648 × 10- 23
A) 3.134 × 1025
B) 1.424 × 1024
C) 7.122 × 1023
D) 5.206 × 1024
E) 8.648 × 10- 23

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
57
The combustion of propane (C3H8 ) in the presence of excess oxygen yields CO2 and H2O:
C3H8 (g) + 5O2 (g) - 3CO2 (g) + 4H2O (g)
When 7.3 g of C3H8 burns in the presence of excess O2, _ g of CO2 is produced.
C3H8 (g) + 5O2 (g) - 3CO2 (g) + 4H2O (g)
When 7.3 g of C3H8 burns in the presence of excess O2, _ g of CO2 is produced.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
58
How many moles of sodium carbonate contain 1.773 × 1017 carbon atoms?
A) 2.945 × 10- 7
B) 8.836 × 10- 7
C) 5.890 × 10- 7
D) 9.817 × 10- 8
E) 1.473 × 10- 7
A) 2.945 × 10- 7
B) 8.836 × 10- 7
C) 5.890 × 10- 7
D) 9.817 × 10- 8
E) 1.473 × 10- 7

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
59
A compound was found to contain 90.6% lead (Pb) and 9.4% oxygen. The empirical formula for this compound is .

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
60
If 294 grams of FeS2 is allowed to react with 176 grams of O2 according to the following equation, how many grams of Fe2O3 are produced? 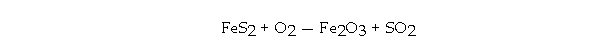
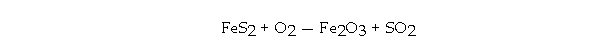

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
61
A certain alcohol contains only three elements, carbon, hydrogen, and oxygen. Combustion of a 50.00 gram sample of the alcohol produced 95.50 grams of CO2 and 58.70 grams of H2O. What is the empirical formula of the alcohol?

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
62
What is the empirical formula of a compound that contains 27.0% S, 13.4% O, and 59.6% Cl by mass?
A) SOCl2
B) S2OCl
C) SOCl
D) SO2Cl
E) ClSO4
A) SOCl2
B) S2OCl
C) SOCl
D) SO2Cl
E) ClSO4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
63
When the following equation is balanced, the coefficient of H2O is _ . 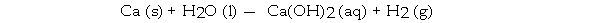
A) 4
B) 1
C) 5
D) 3
E) 2
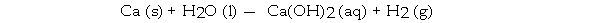
A) 4
B) 1
C) 5
D) 3
E) 2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
64
When the following equation is balanced, the coefficient of H3PO4 is _ . 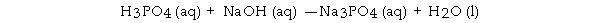
A) 2
B) 0
C) 1
D) 3
E) 4
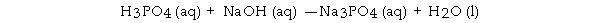
A) 2
B) 0
C) 1
D) 3
E) 4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
65
There are _ mol of carbon atoms in 4 mol of dimethylsulfoxide (C2H6SO).
A) 2
B) 8
C) 4
D) 6
E) 3
A) 2
B) 8
C) 4
D) 6
E) 3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
66
When the following equation is balanced, the coefficient of Al is . 
A) 3
B) 1
C) 5
D) 4
E) 2

A) 3
B) 1
C) 5
D) 4
E) 2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
67
The combustion of ammonia in the presence of excess oxygen yields NO2 and H2O:
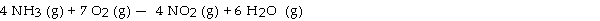
The combustion of 28.8 g of ammonia consumes g of oxygen.
A) 108
B) 94.9
C) 28.8
D) 54.1
E) 15.3
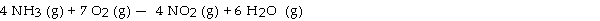
The combustion of 28.8 g of ammonia consumes g of oxygen.
A) 108
B) 94.9
C) 28.8
D) 54.1
E) 15.3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
68
When the following equation is balanced, the coefficient of H2SO4 is _ . 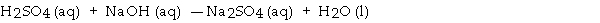
A) 2
B) 3
C) 0.5
D) 1
E) 4
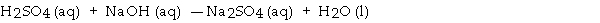
A) 2
B) 3
C) 0.5
D) 1
E) 4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
69
Complete and balance the following reaction, given that elemental rubidium reacts with elemental sulfur to form Rb2S (s).
Na (s) + S (s) -
Na (s) + S (s) -

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
70
The combustion of carbon disulfide in the presence of excess oxygen yields carbon dioxide and sulfur dioxide:
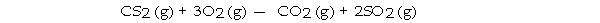
The combustion of 15 g of CS2 in the presence of excess oxygen yields g of SO2.
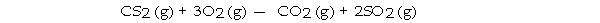
The combustion of 15 g of CS2 in the presence of excess oxygen yields g of SO2.

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
71
When the following equation is balanced, the coefficient of HCl is . 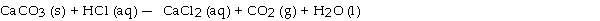
A) 2
B) 1
C) 0
D) 4
E) 3
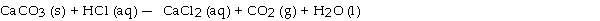
A) 2
B) 1
C) 0
D) 4
E) 3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
72
How many moles of magnesium oxide are produced by the reaction of 3.82 g of magnesium nitride with 7.73 g of water? 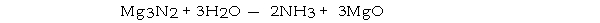
A) 0.0378
B) 0.113
C) 0.429
D) 4.57
E) 0.0756
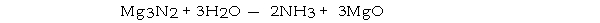
A) 0.0378
B) 0.113
C) 0.429
D) 4.57
E) 0.0756

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
73
A compound is composed of only C, H, and O. The combustion of a 0.519- g sample of the compound yields 1.24 g of CO2 and 0.255 g of H2O. What is the empirical formula of the compound?
A) C6H6O
B) CH3O
C) C3H3O
D) C2H6O2
E) C2H6O5
A) C6H6O
B) CH3O
C) C3H3O
D) C2H6O2
E) C2H6O5

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
74
Write the balanced equation for the reaction that occurs when methanol, CH3OH (l), is burned in air. What is the coefficient of methanol in the balanced equation?
A) 1
B) 2
C) 3
D) 4
E) 3/2
A) 1
B) 2
C) 3
D) 4
E) 3/2

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
75
A 2.25- g sample of magnesium nitrate, Mg(NO3)2, contains mol of this compound.
A) 0.0261
B) 65.8
C) 0.0152
D) 148.3
E) 38.4
A) 0.0261
B) 65.8
C) 0.0152
D) 148.3
E) 38.4

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
76
Under appropriate conditions, nitrogen and hydrogen undergo a combination reaction to yield ammonia:
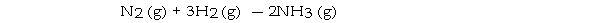
A 9.3- g sample of hydrogen requires
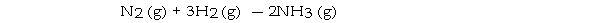
A 9.3- g sample of hydrogen requires

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
77
Determine the mass percent (to the hundredth's place) of H in sodium bicarbonate (NaHCO3).

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
78
A compound that is composed of only carbon and hydrogen contains 80.0% C and 20.0% H by mass. What is the empirical formula of the compound?
A) C2H6
B) C20H60
C) CH4
D) C7H20
E) CH3
A) C2H6
B) C20H60
C) CH4
D) C7H20
E) CH3

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
79
A compound contains 40.0% C, 6.71% H, and 53.29% O by mass. The molecular weight of the compound is 60.05 amu. The molecular formula of this compound is _ _.
A) C2H3O4
B) CHO2
C) C2H4O2
D) C2H2O4
E) CH2O
A) C2H3O4
B) CHO2
C) C2H4O2
D) C2H2O4
E) CH2O

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck
80
Lead (II) carbonate decomposes to give lead (II) oxide and carbon dioxide:

How many grams of lead (II) oxide will be produced by the decomposition of 2.50 g of lead (II) carbonate?
A) 0.00936
B) 0.41
C) 2.61
D) 2.09
E) 2.50

How many grams of lead (II) oxide will be produced by the decomposition of 2.50 g of lead (II) carbonate?
A) 0.00936
B) 0.41
C) 2.61
D) 2.09
E) 2.50

Unlock Deck
Unlock for access to all 134 flashcards in this deck.
Unlock Deck
k this deck


