Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
When the following equation is balanced, the coefficients are . 
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
D
The formula weight of potassium phosphate (K3PO4) is _ amu.
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
A
Lithium and nitrogen react in a combination reaction to produce lithium nitride:
 How many moles of N2 are needed to react with 0.500 mol of lithium?
How many moles of N2 are needed to react with 0.500 mol of lithium?
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
B
A sample of CH4O with a mass of 32.0 g contains molecules of CH4O.
(Multiple Choice)
4.7/5  (28)
(28)
How many oxygen atoms are there in 52.06 g of carbon dioxide?
(Multiple Choice)
4.8/5  (34)
(34)
The balanced equation for the decomposition of sodium azide is .
(Multiple Choice)
4.8/5  (40)
(40)
A 22.5- g sample of ammonium carbonate contains mol of ammonium ions.
(Multiple Choice)
4.8/5  (29)
(29)
Pentacarbonyliron (Fe(CO)5) reacts with phosphorous trifluoride (PF3) and hydrogen, releasing carbon monoxide:
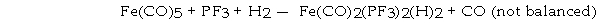 The reaction of 5.0 mol of Fe(CO)5, 8.0 mol of PF3 and 6.0 mol of H2 will release _ _ mol of CO.
The reaction of 5.0 mol of Fe(CO)5, 8.0 mol of PF3 and 6.0 mol of H2 will release _ _ mol of CO.
(Multiple Choice)
5.0/5  (25)
(25)
Calculate the percentage by mass of nitrogen in PtCl2(NH3)2.
(Multiple Choice)
4.7/5  (39)
(39)
How many moles of magnesium oxide are produced by the reaction of 3.82 g of magnesium nitride with 7.73 g of water? 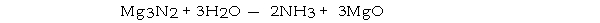
(Multiple Choice)
4.9/5  (36)
(36)
A sample of CH2F2 with a mass of 19 g contains _ atoms of F.
(Multiple Choice)
4.7/5  (33)
(33)
There are _ mol of carbon atoms in 4 mol of dimethylsulfoxide (C2H6SO).
(Multiple Choice)
4.7/5  (39)
(39)
When the following equation is balanced, the coefficient of H2 is _ . 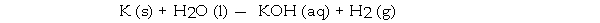
(Multiple Choice)
4.8/5  (34)
(34)
The formula of nitrobenzene is C6H5NO2. The molecular weight of this compound is amu.
(Multiple Choice)
4.8/5  (36)
(36)
What is the coefficient of O2 when the following equation is completed and balanced?
C4H8O2 + O2 -
(Multiple Choice)
4.8/5  (33)
(33)
Determine the mass percent (to the hundredth's place) of H in sodium bicarbonate (NaHCO3).
(Short Answer)
4.7/5  (35)
(35)
If 294 grams of FeS2 is allowed to react with 176 grams of O2 according to the following equation, how many grams of Fe2O3 are produced? 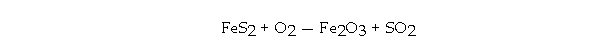
(Short Answer)
4.8/5  (27)
(27)
Automotive air bags inflate when sodium azide decomposes explosively to its constituent elements:
 How many grams of sodium azide are required to produce 18.0 g of nitrogen?
How many grams of sodium azide are required to produce 18.0 g of nitrogen?
(Multiple Choice)
4.9/5  (40)
(40)
Showing 1 - 20 of 134
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)