Deck 23: Metals and Metallurgy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/112
Play
Full screen (f)
Deck 23: Metals and Metallurgy
1
A small amount of barium chloride solution is added to a blue solution. A white precipitate forms. The blue solution contains
A) CoCl2
B) CuSO4
C) NiSO4
D) Co(NO3)3
E) CuCl2
A) CoCl2
B) CuSO4
C) NiSO4
D) Co(NO3)3
E) CuCl2
CuSO4
2
What is the typical effect of the addition of an interstitial element on the properties of a metal?
A) increased surface luster
B) increase in malleability and corrosion resistance
C) decrease in conductivity and increase in brittleness
D) decrease in melting point and increase in ductility
E) increase in hardness and strength, decrease in ductility
A) increased surface luster
B) increase in malleability and corrosion resistance
C) decrease in conductivity and increase in brittleness
D) decrease in melting point and increase in ductility
E) increase in hardness and strength, decrease in ductility
increase in hardness and strength, decrease in ductility
3
Which one of the following compounds is yellow?
A) CCl2
B) K2CrO4
C) (NH4)2Cr2O7
D) Cr(NO3)3
E) CrCl3
A) CCl2
B) K2CrO4
C) (NH4)2Cr2O7
D) Cr(NO3)3
E) CrCl3
K2CrO4
4
The lithosphere is the
A) deepest part of the ocean.
B) portion of the atmosphere closest to the Earth.
C) portion of the atmosphere furthest from the Earth.
D) solid surface of the Earth.
E) molten core of the Earth.
A) deepest part of the ocean.
B) portion of the atmosphere closest to the Earth.
C) portion of the atmosphere furthest from the Earth.
D) solid surface of the Earth.
E) molten core of the Earth.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
5
What are the products of the following (unbalanced) leaching process? 
A) Au(CN)4- (aq) and OH- (aq)
B) Au(CN)2- (aq) and OH- (aq)
C) AuCN (s) and OH- (aq)
D) AuCN (s) and H2O2 (aq)
E) Au(CN)3 (s) and OH- (aq)

A) Au(CN)4- (aq) and OH- (aq)
B) Au(CN)2- (aq) and OH- (aq)
C) AuCN (s) and OH- (aq)
D) AuCN (s) and H2O2 (aq)
E) Au(CN)3 (s) and OH- (aq)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
6
The purpose of burning coke in a blast furnace is
A) to produce hydrogen gas.
B) to produce carbon monoxide gas.
C) to produce reducing gases.
D) to produce heat.
E) all of the above
A) to produce hydrogen gas.
B) to produce carbon monoxide gas.
C) to produce reducing gases.
D) to produce heat.
E) all of the above

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
7
The purpose of a converter in steel production is
A) to remove impurity elements by oxidation
B) to allow the addition of nitrogen for increased strength
C) to reduce the iron in the ore to elemental
D) to allow the formation of phosphides within the metal for added corrosion resistance
E) to allow slow solidification of the molten metal so it will purify as it crystallizes
A) to remove impurity elements by oxidation
B) to allow the addition of nitrogen for increased strength
C) to reduce the iron in the ore to elemental
D) to allow the formation of phosphides within the metal for added corrosion resistance
E) to allow slow solidification of the molten metal so it will purify as it crystallizes

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
8
Why do aqueous solutions of Cr2+ usually appear violet instead of blue?
A) The intensity of the color is so low it appears violet instead of blue.
B) Chromium(II) rapidly forms a complex ion with water and that ion is violet- colored.
C) Chromium(II) reacts with water to form a hydroxide- containing complex ion that is violet- colored.
D) Chromium(II) is rapidly oxidized to chromium(III) by atmospheric oxygen.
E) Water usually contains enough chloride ion to form a complex ion containing chloride and that substance is violet- colored.
A) The intensity of the color is so low it appears violet instead of blue.
B) Chromium(II) rapidly forms a complex ion with water and that ion is violet- colored.
C) Chromium(II) reacts with water to form a hydroxide- containing complex ion that is violet- colored.
D) Chromium(II) is rapidly oxidized to chromium(III) by atmospheric oxygen.
E) Water usually contains enough chloride ion to form a complex ion containing chloride and that substance is violet- colored.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
9
A basic slag is needed in steelmaking to
A) remove SiO2 as silicates.
B) reduce any nitrogen- containing compounds to N2.
C) oxidize any carbon- containing compounds to CO2.
D) react with any BrØnsted- Lowry acids present.
E) provide CaO to remove phosphorus oxides as Ca3(PO4)2.
A) remove SiO2 as silicates.
B) reduce any nitrogen- containing compounds to N2.
C) oxidize any carbon- containing compounds to CO2.
D) react with any BrØnsted- Lowry acids present.
E) provide CaO to remove phosphorus oxides as Ca3(PO4)2.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
10
The undesirable material that is separated from an ore during the concentration process is called
)
A) flocculent
B) silicate
C) gangue
D) slag
E) leachate
)
A) flocculent
B) silicate
C) gangue
D) slag
E) leachate

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
11
The lanthanide contraction is responsible for the fact that
A) Zr and Zn have similar oxidation states.
B) Zr and Hf have the same oxidation states.
C) Zr and Y have about the same radius.
D) Zr and Hf have about the same radius.
E) Zr and Nb have similar oxidation states.
A) Zr and Zn have similar oxidation states.
B) Zr and Hf have the same oxidation states.
C) Zr and Y have about the same radius.
D) Zr and Hf have about the same radius.
E) Zr and Nb have similar oxidation states.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
12
Which element is typically not added to steel to modify its properties?
A) vanadium
B) carbon
C) nickel
D) chromium
E) nitrogen
A) vanadium
B) carbon
C) nickel
D) chromium
E) nitrogen

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
13
Of the following, which is a bulk property?
A) melting point
B) atomic number
C) ionization energy
D) atomic radius
E) electron configuration
A) melting point
B) atomic number
C) ionization energy
D) atomic radius
E) electron configuration

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
14
Calcium chloride is added to sodium chloride prior to melting it for electrolysis in a Downs cell
)
A) to provide calcium ions which serve as the cathode
B) to lower the melting point
C) to increase the concentration of chloride ions
D) to make the chloride ions easier to oxidize
E) to make the sodium ions easier to reduce
)
A) to provide calcium ions which serve as the cathode
B) to lower the melting point
C) to increase the concentration of chloride ions
D) to make the chloride ions easier to oxidize
E) to make the sodium ions easier to reduce

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
15
A mixture of oxygen and argon are blown through molten iron that is produced in a blast furnace for the purpose of
A) removing carbon, sulfur, and other impurities.
B) oxidizing the iron before it cools.
C) cooling the molten iron.
D) completing the reduction of iron.
E) removing other metals.
A) removing carbon, sulfur, and other impurities.
B) oxidizing the iron before it cools.
C) cooling the molten iron.
D) completing the reduction of iron.
E) removing other metals.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
16
Part of the Bayer process involves the digestion of crushed ore in concentrated aqueous sodium hydroxide. This process carried out at high pressure .
A) to prevent formation of aluminum hydroxide
B) to prevent boiling
C) to accelerate formation of iron hydroxide
D) to lower the boiling temperature of the mixture
E) to prevent formation of iron hydroxide
A) to prevent formation of aluminum hydroxide
B) to prevent boiling
C) to accelerate formation of iron hydroxide
D) to lower the boiling temperature of the mixture
E) to prevent formation of iron hydroxide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
17
Silicon, doped with a group 5A element forms a(n) - type semiconductor. Silicon doped with a group 3A element forms a(n) - type semiconductor.
A) n, n
B) n, p
C) p, n
D) p, p
E) None of the above is correct.
A) n, n
B) n, p
C) p, n
D) p, p
E) None of the above is correct.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
18
The product in the Hall electrometallurgical process is
A) iron.
B) copper.
C) gold.
D) silver.
E) aluminum.
A) iron.
B) copper.
C) gold.
D) silver.
E) aluminum.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
19
A deposit that contains a metal in economically exploitable quantities is called a(n) _.
A) vein
B) metal
C) ore
D) mineral
E) comstock
A) vein
B) metal
C) ore
D) mineral
E) comstock

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
20
In the Bayer process, what is the product of calcination of the aluminum hydroxide recovered from the digestion in base?
A) Al(OH)4- (aq)
B) Al · xH2O (s)
C) Al (s)
D) Al(OH)3 (s)
E) Al2O3 (s)
A) Al(OH)4- (aq)
B) Al · xH2O (s)
C) Al (s)
D) Al(OH)3 (s)
E) Al2O3 (s)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is not an alloy?
A) steel
B) dental amalgam
C) ceramic
D) brass
E) sterling silver
A) steel
B) dental amalgam
C) ceramic
D) brass
E) sterling silver

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
22
Which one of the following copper compounds is black in color?
A) Cu(H2O)42+
B) CuO
C) CuI
D) CuSO4
E) CuCl2
A) Cu(H2O)42+
B) CuO
C) CuI
D) CuSO4
E) CuCl2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
23
What two oxidation states are more frequently observed in the first transition series than in the third?
A) +5 and +6
B) +3 and +5
C) +3 and +7
D) +2 and +7
E) +2 and +3
A) +5 and +6
B) +3 and +5
C) +3 and +7
D) +2 and +7
E) +2 and +3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
24
Which statement below is true?
A) Most metallic elements are found in the lithosphere in oxidation state zero.
B) There exists little correlation between the abundance of an element in the lithosphere and its commercial extraction and use.
C) The United States has plentiful ore fields of all strategic metals.
D) New mining techniques and relatively untapped ore fields mean that the environmental impacts of mineral extraction will decrease significantly in the future.
E) The most important commercial class of minerals is the silicates.
A) Most metallic elements are found in the lithosphere in oxidation state zero.
B) There exists little correlation between the abundance of an element in the lithosphere and its commercial extraction and use.
C) The United States has plentiful ore fields of all strategic metals.
D) New mining techniques and relatively untapped ore fields mean that the environmental impacts of mineral extraction will decrease significantly in the future.
E) The most important commercial class of minerals is the silicates.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
25
For a substitutional alloy to form, the two metals combined must have similar
A) ionization potential and electron affinity.
B) atomic radii and chemical bonding properties.
C) reduction potential and size.
D) number of valance electrons and electronegativity.
E) band gap and reactivity.
A) ionization potential and electron affinity.
B) atomic radii and chemical bonding properties.
C) reduction potential and size.
D) number of valance electrons and electronegativity.
E) band gap and reactivity.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
26
The respective standard oxidation potentials for Cu -Cu2+, Ni -Ni2+, and Ag -Ag+ are (in V)
- 0)34, +0.28, and - 0.80. Impure copper slabs at the anode are refined electrochemically, affording much purer metallic copper at the cathode. Which statement below is true?
A) Ni is oxidized preferentially over Cu, and Ni2+ is reduced much less readily than Cu2+, so Ni is separated as Ni2+ in the electrolyte solution.
B) Cu is oxidized preferentially over both Ni and Ag, so both Ni and Ag metals are separated as sludges below the anode.
C) Ag is oxidized preferentially over Cu, and Ag+ is reduced much less readily than Cu2+, so Ag is separated as Ag+ in the electrolyte solution.
D) Ag is oxidized preferentially over Cu, and Ag+ is reduced much more readily than Cu2+, so Ag plates out with Cu at the cathode and cannot readily be removed from impure copper.
E) Both Ni and Ag are oxidized preferentially over Cu and Ni2+, and Ag+ is reduced much less readily than Cu2+, so Ni and Ag are separated as Ni2+ and Ag+ in the electrolyte solution.
- 0)34, +0.28, and - 0.80. Impure copper slabs at the anode are refined electrochemically, affording much purer metallic copper at the cathode. Which statement below is true?
A) Ni is oxidized preferentially over Cu, and Ni2+ is reduced much less readily than Cu2+, so Ni is separated as Ni2+ in the electrolyte solution.
B) Cu is oxidized preferentially over both Ni and Ag, so both Ni and Ag metals are separated as sludges below the anode.
C) Ag is oxidized preferentially over Cu, and Ag+ is reduced much less readily than Cu2+, so Ag is separated as Ag+ in the electrolyte solution.
D) Ag is oxidized preferentially over Cu, and Ag+ is reduced much more readily than Cu2+, so Ag plates out with Cu at the cathode and cannot readily be removed from impure copper.
E) Both Ni and Ag are oxidized preferentially over Cu and Ni2+, and Ag+ is reduced much less readily than Cu2+, so Ni and Ag are separated as Ni2+ and Ag+ in the electrolyte solution.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
27
The hydrated nickel(II) ion is
A) green.
B) yellow.
C) orange.
D) colorless.
E) blue.
A) green.
B) yellow.
C) orange.
D) colorless.
E) blue.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
28
The anode sludges from copper refining are important sources of what metal(s)?
A) all of the above
B) gold
C) both a and b
D) silver
E) aluminum
A) all of the above
B) gold
C) both a and b
D) silver
E) aluminum

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
29
Alloys generally differ from compounds in that
A) the former always contain some iron.
B) the atomic ratios of the constituent elements in the former are not fixed and may vary over a wide range.
C) the former never contain a transition element.
D) the former always have semiconductor properties.
E) the former always contain some carbon.
A) the former always contain some iron.
B) the atomic ratios of the constituent elements in the former are not fixed and may vary over a wide range.
C) the former never contain a transition element.
D) the former always have semiconductor properties.
E) the former always contain some carbon.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
30
Roasting of the disulfide of molybdenum in O2 produces which products?
A) MoO3 (s) + SO3 (g)
B) MoO4 (s) + SO2 (g)
C) Mo (s) + SO2 ( g)
D) MoO3 (s) + SO2 (g)
E) Mo (s) + MoS (s) + SO2 (g)
A) MoO3 (s) + SO3 (g)
B) MoO4 (s) + SO2 (g)
C) Mo (s) + SO2 ( g)
D) MoO3 (s) + SO2 (g)
E) Mo (s) + MoS (s) + SO2 (g)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
31
A substance with unpaired electrons will be
A) slightly attracted to a magnet.
B) slightly repelled by a magnet.
C) brightly colored.
D) permanently magnetic.
E) nonmetallic.
A) slightly attracted to a magnet.
B) slightly repelled by a magnet.
C) brightly colored.
D) permanently magnetic.
E) nonmetallic.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
32
In the Bayer process, the purpose of lowering the pH after the digested ore has been filtered is
)
A) the dissolve the remaining ore
B) to precipitate the iron oxide
C) to precipitate the aluminum hydroxide
D) to precipitate the iron hydroxide
E) to dissolve the remaining impurities
)
A) the dissolve the remaining ore
B) to precipitate the iron oxide
C) to precipitate the aluminum hydroxide
D) to precipitate the iron hydroxide
E) to dissolve the remaining impurities

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
33
Reaction of iron with which one of the following acids will result in the direct production of Fe3+?
A) HI
B) dilute H2SO4
C) HCl
D) HNO3
E) CH3COOH
A) HI
B) dilute H2SO4
C) HCl
D) HNO3
E) CH3COOH

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
34
A mineral is
A) metal in its elemental form.
B) a vitamin.
C) a transition metal ion.
D) a solid inorganic compound that contains one or more metals.
E) source of carbon.
A) metal in its elemental form.
B) a vitamin.
C) a transition metal ion.
D) a solid inorganic compound that contains one or more metals.
E) source of carbon.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
35
What happens to the silicon that is a contaminant in crude iron in a converter?
A) it is converted to the tetrafluoride that bubbles out as a gas.
B) It is precipitated as the carbide.
C) It is precipitated as sodium silicate.
D) It is precipitated as iron silicate.
E) It is converted to silicon dioxide and becomes part of the slag.
A) it is converted to the tetrafluoride that bubbles out as a gas.
B) It is precipitated as the carbide.
C) It is precipitated as sodium silicate.
D) It is precipitated as iron silicate.
E) It is converted to silicon dioxide and becomes part of the slag.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
36
When copper(II) hydroxide is heated, _ and _ are formed.
A) CuH2 (s) + O2 (g)
B) CuOH (s) + H2O (g)
C) Cu (s), OH- (g)
D) Cu (s), H2O (l)
E) CuO (s), H2O (l)
A) CuH2 (s) + O2 (g)
B) CuOH (s) + H2O (g)
C) Cu (s), OH- (g)
D) Cu (s), H2O (l)
E) CuO (s), H2O (l)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
37
Intermetallic compounds are examples of
A) homogeneous alloys.
B) solution alloys.
C) ionic compounds.
D) heterogeneous alloys.
E) interstitial alloys.
A) homogeneous alloys.
B) solution alloys.
C) ionic compounds.
D) heterogeneous alloys.
E) interstitial alloys.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
38
Heterogeneous alloys
A) have properties that depend on the manner in which the solid is formed.
B) have properties that depend on composition.
C) have properties that depend on the manner in which the melt is solidified.
D) do not have uniform composition throughout.
E) All of the above are true.
A) have properties that depend on the manner in which the solid is formed.
B) have properties that depend on composition.
C) have properties that depend on the manner in which the melt is solidified.
D) do not have uniform composition throughout.
E) All of the above are true.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
39
An advantage to leaching gold with aqueous solutions of cyanide ion is that
A) the gold recovered in this way has higher purity than gold recovered in other ways.
B) the process requires only one step.
C) the method can be used to locate underground gold deposits.
D) gold can be recovered from very low- grade ores by this method.
E) the process is environmentally safe.
A) the gold recovered in this way has higher purity than gold recovered in other ways.
B) the process requires only one step.
C) the method can be used to locate underground gold deposits.
D) gold can be recovered from very low- grade ores by this method.
E) the process is environmentally safe.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
40
Gold and the platinum group metals are found in nature in metallic form because
A) they are relatively abundant.
B) they are soluble in water.
C) they are relatively inert.
D) they are solids at room temperature.
E) they are highly reactive.
A) they are relatively abundant.
B) they are soluble in water.
C) they are relatively inert.
D) they are solids at room temperature.
E) they are highly reactive.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
41
During roasting, the metal reacts with
A) oxygen.
B) iron.
C) sulfur.
D) the furnace atmosphere.
E) carbon monoxide.
A) oxygen.
B) iron.
C) sulfur.
D) the furnace atmosphere.
E) carbon monoxide.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
42
Why is either pure oxygen or oxygen diluted with argon used in a converter instead of air?
A) The oxygen concentration is too low to function efficiently at removing impurities.
B) The carbon monoxide in air reacts with the iron to form a volatile, and toxic, iron carbonyl.
C) The nitrogen in air will react with iron to form iron nitride that will make the iron brittle.
D) Because it's cheaper.
E) The carbon dioxide in air will cause the iron to oxidize and form rust.
A) The oxygen concentration is too low to function efficiently at removing impurities.
B) The carbon monoxide in air reacts with the iron to form a volatile, and toxic, iron carbonyl.
C) The nitrogen in air will react with iron to form iron nitride that will make the iron brittle.
D) Because it's cheaper.
E) The carbon dioxide in air will cause the iron to oxidize and form rust.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
43
Shape memory alloys .
A) in their lower temperature phase have a flexible arrangement between atoms
B) in their higher temperature phase have strong and fixed bonds between atoms
C) change their structure as the temperature changes
D) are more pliable when cold than when warm
E) all of the above
A) in their lower temperature phase have a flexible arrangement between atoms
B) in their higher temperature phase have strong and fixed bonds between atoms
C) change their structure as the temperature changes
D) are more pliable when cold than when warm
E) all of the above

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following are not commonly used as sources of metals?
A) silicates
B) oxides
C) sulfides
D) carbonates
E) All of the above are commonly used sources of metals.
A) silicates
B) oxides
C) sulfides
D) carbonates
E) All of the above are commonly used sources of metals.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
45
Which one of the following is false concerning the Bayer Process?
A) It is a hydrometallurgical process.
B) It results in the separation of aluminum from iron and silicon.
C) It involves treatment of bauxite with cold, dilute sodium hydroxide solution.
D) It is used to purify bauxite.
E) In the process, aluminum is converted to a soluble aluminate ion.
A) It is a hydrometallurgical process.
B) It results in the separation of aluminum from iron and silicon.
C) It involves treatment of bauxite with cold, dilute sodium hydroxide solution.
D) It is used to purify bauxite.
E) In the process, aluminum is converted to a soluble aluminate ion.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
46
A disadvantage to leaching gold with aqueous solutions of cyanide ion is that
A) it is a complicated process requiring more than two dozen separate steps.
B) it requires the use of ores with a very high concentration of gold.
C) sodium cyanide is very expensive.
D) the method is a potential environmental hazard.
E) the method does not produce gold of high enough purity.
A) it is a complicated process requiring more than two dozen separate steps.
B) it requires the use of ores with a very high concentration of gold.
C) sodium cyanide is very expensive.
D) the method is a potential environmental hazard.
E) the method does not produce gold of high enough purity.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
47
The hydrated manganese(II) ion is
A) blue.
B) violet.
C) orange.
D) colorless.
E) yellow.
A) blue.
B) violet.
C) orange.
D) colorless.
E) yellow.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
48
The major impurities found in bauxite are .
A) Fe and H2O
B) SiO2 and Fe2O3
C) Al2O3 and H2O
D) carbon and salicylic acid
E) Al2O3 and OH-
A) Fe and H2O
B) SiO2 and Fe2O3
C) Al2O3 and H2O
D) carbon and salicylic acid
E) Al2O3 and OH-

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
49
An alloy is a
A) a mineral containing two or more metals.
B) pure metal.
C) heterogeneous mixture of two metals.
D) metallic material that is composed of two or more elements.
E) nonmetal with some properties of a metal.
A) a mineral containing two or more metals.
B) pure metal.
C) heterogeneous mixture of two metals.
D) metallic material that is composed of two or more elements.
E) nonmetal with some properties of a metal.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following equations represents the roasting of an ore?
A) All of these represent roasting processes.
B) PbO (s) + CO (g)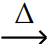 Pb (l) + CO2 (g)
Pb (l) + CO2 (g)
C) HgS (s) + O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11Hg (g) + SO2 (g)
D) 2ZnS (s) + 3O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_112ZnO (s) + 2SO2 (g)
E) 2MoS2 (s) + 7O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_112MoO3 (s) + 4SO2 (g)
A) All of these represent roasting processes.
B) PbO (s) + CO (g)
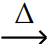 Pb (l) + CO2 (g)
Pb (l) + CO2 (g)C) HgS (s) + O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11Hg (g) + SO2 (g)
D) 2ZnS (s) + 3O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_112ZnO (s) + 2SO2 (g)
E) 2MoS2 (s) + 7O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_112MoO3 (s) + 4SO2 (g)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
51
The Hall process is
A) the pyrometallurgic process used to produce aluminum.
B) the pyrometallurgic process used to produce iron.
C) the electrolytic process used to produce iron.
D) the hydrometallurgic process to produce aluminum.
E) the electrolytic process used to produce aluminum.
A) the pyrometallurgic process used to produce aluminum.
B) the pyrometallurgic process used to produce iron.
C) the electrolytic process used to produce iron.
D) the hydrometallurgic process to produce aluminum.
E) the electrolytic process used to produce aluminum.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
52
The molecular- orbital model for Ge shows it to be
A) an insulator, because all the lower energy band orbitals are filled and the gap between the lower and higher bands is large.
B) a conductor, because its lower energy band orbitals are only partially filled.
C) a conductor, because all the lower energy band orbitals are filled and the gap between the lower and higher bands is large.
D) a semiconductor, because the gap between the filled lower and empty higher energy bands is relatively small.
E) a semiconductor, because the gap between the filled lower and empty higher energy bands is large.
A) an insulator, because all the lower energy band orbitals are filled and the gap between the lower and higher bands is large.
B) a conductor, because its lower energy band orbitals are only partially filled.
C) a conductor, because all the lower energy band orbitals are filled and the gap between the lower and higher bands is large.
D) a semiconductor, because the gap between the filled lower and empty higher energy bands is relatively small.
E) a semiconductor, because the gap between the filled lower and empty higher energy bands is large.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
53
Which statement about steel is false?
A) It can have different percentage of carbon.
B) It is a polymer.
C) It is an alloy of iron.
D) In can be made so it resists rust.
E) none of the above
A) It can have different percentage of carbon.
B) It is a polymer.
C) It is an alloy of iron.
D) In can be made so it resists rust.
E) none of the above

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
54
Which mineral contains aluminum?
A) malachite
B) bauxite
C) cinnabar
D) magnetite
E) galena
A) malachite
B) bauxite
C) cinnabar
D) magnetite
E) galena

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
55
If the electronic structure of a solid substance consists of a valence band that is completely filled with electrons and there is a large energy gap to the next set of orbitals, then this substance will be a(n) _ .
A) semiconductor
B) conductor
C) alloy
D) nonmetal
E) insulator
A) semiconductor
B) conductor
C) alloy
D) nonmetal
E) insulator

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
56
Which one of the following metallic elements is most likely to be found as the free metal in nature?
A) Au
B) Al
C) Li
D) Ca
E) Fe
A) Au
B) Al
C) Li
D) Ca
E) Fe

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
57
Hydrometallurgy is
A) the use of aqueous solutions to extract metals from their ores.
B) the use of water to cool molten metals.
C) the use of high temperature processes to concentrate and refine metals.
D) the use of high temperature processes to make alloys.
E) the use of water to locate underground ore deposits.
A) the use of aqueous solutions to extract metals from their ores.
B) the use of water to cool molten metals.
C) the use of high temperature processes to concentrate and refine metals.
D) the use of high temperature processes to make alloys.
E) the use of water to locate underground ore deposits.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
58
Smelting is
A) thermal decomposition of an ore with elimination of a gaseous product.
B) cooling a molten metal to make it solidify.
C) heating an ore to make it react with a gas in a furnace.
D) addition of calcium to a molten ore.
E) melting and subsequent reaction of molten ores resulting in the formation of layers.
A) thermal decomposition of an ore with elimination of a gaseous product.
B) cooling a molten metal to make it solidify.
C) heating an ore to make it react with a gas in a furnace.
D) addition of calcium to a molten ore.
E) melting and subsequent reaction of molten ores resulting in the formation of layers.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
59
Which property of metals cannot be explained with the electron- sea model?
A) shine
B) trends in melting points
C) high thermal connectivity
D) high electric connectivity
E) malleability and ductility
A) shine
B) trends in melting points
C) high thermal connectivity
D) high electric connectivity
E) malleability and ductility

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
60
Sodium metal cannot be produced by electrolysis of an aqueous solution of sodium chloride because
A) the iron cathode would be corroded by the salt water.
B) water is more easily reduced than sodium ions.
C) the carbon anode is more easily reduced than sodium ions.
D) the production of chlorine gas interferes with the reduction of sodium ions.
E) water is more easily oxidized than sodium metal.
A) the iron cathode would be corroded by the salt water.
B) water is more easily reduced than sodium ions.
C) the carbon anode is more easily reduced than sodium ions.
D) the production of chlorine gas interferes with the reduction of sodium ions.
E) water is more easily oxidized than sodium metal.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
61
What two metals are alloyed to produce sterling silver?

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
62
Anhydrous aluminum oxide is dissolved in molten cryolite rather than simply melted because
__________
A) the cryolite provides a source of sodium ions.
B) the melting point of pure, anhydrous aluminum oxide is too high.
C) the cryolite provides the necessary fluoride ions.
D) the cryolite actually provides the aluminum that is to be reduced.
E) in pure, molten Al2O3, the aluminum would be oxidized rather than reduced.
__________
A) the cryolite provides a source of sodium ions.
B) the melting point of pure, anhydrous aluminum oxide is too high.
C) the cryolite provides the necessary fluoride ions.
D) the cryolite actually provides the aluminum that is to be reduced.
E) in pure, molten Al2O3, the aluminum would be oxidized rather than reduced.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
63
Draw a diagram of the short- hand ground state electron configuration of zinc.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
64
Give the name and formula of the two important ores of iron.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
65
Most of the metals in the third transition series have about the same atomic radius as the elements above them in second transition series. This is a result of .

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
66
What is produced when a carbonate is calcined?
A) the free metal and carbon dioxide
B) the free metal and sodium carbonate
C) water and the metal hydride
D) the free metal and sulfur dioxide
E) the metal oxide and carbon dioxide
A) the free metal and carbon dioxide
B) the free metal and sodium carbonate
C) water and the metal hydride
D) the free metal and sulfur dioxide
E) the metal oxide and carbon dioxide

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
67
Which one of the following is a property of most metals?
A) brittleness
B) high electronegativity
C) low melting point
D) thermal conductivity
E) acidic oxides
A) brittleness
B) high electronegativity
C) low melting point
D) thermal conductivity
E) acidic oxides

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
68
Steel is
A) pure iron.
B) a liquid at room temperature.
C) oxidized iron.
D) an alloy of iron.
E) a mixture of iron and silver.
A) pure iron.
B) a liquid at room temperature.
C) oxidized iron.
D) an alloy of iron.
E) a mixture of iron and silver.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
69
A slag is formed when a basic metal oxide reacts with molten _ at high temperatures.
A) CaO
B) Fe2O3
C) CaSiO3
D) CO
E) SiO2
A) CaO
B) Fe2O3
C) CaSiO3
D) CO
E) SiO2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
70
Which one of the following is not true about transition metals?
A) They frequently have more than one common oxidation state.
B) Their compounds frequently exhibit magnetic properties.
C) They are found in the d- block of the periodic table.
D) Their compounds are frequently colored.
E) They typically have low melting points.
A) They frequently have more than one common oxidation state.
B) Their compounds frequently exhibit magnetic properties.
C) They are found in the d- block of the periodic table.
D) Their compounds are frequently colored.
E) They typically have low melting points.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
71
A material that contains more than one element and has the characteristic properties of metals is called a (an) .

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
72
What is the purpose of adding zinc powder to a solution of Au(CN)2- ?
A) to precipitate the cyanide- gold complex
B) to form a gold- zinc alloy
C) to reduce the gold in the cyanide complex to gold metal
D) to precipitate the cyanide ion
E) to oxidize the gold in the cyanide complex to gold metal
A) to precipitate the cyanide- gold complex
B) to form a gold- zinc alloy
C) to reduce the gold in the cyanide complex to gold metal
D) to precipitate the cyanide ion
E) to oxidize the gold in the cyanide complex to gold metal

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
73
Write the two reactions that are used to control the temperature in a blast furnace and indicate which provides heat and which cools.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following equations represents a calcination?
A) PbCO3 (s)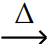 PbO (s) + CO2 (g)
PbO (s) + CO2 (g)
B) CaO (l) + SiO2 (l) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11CaSiO3 (l)
C) HgS (s) + O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11Hg (g) + SO2 (g)
D) PbO (s) + CO (g)11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11Pb (l) + CO2 (g)
E) All of the above are calcination processes.
A) PbCO3 (s)
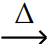 PbO (s) + CO2 (g)
PbO (s) + CO2 (g)B) CaO (l) + SiO2 (l) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11CaSiO3 (l)
C) HgS (s) + O2 (g) 11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11Hg (g) + SO2 (g)
D) PbO (s) + CO (g)11ec835a_37ed_5a1a_b7a5_d3a2a63a892c_TB34225555_11Pb (l) + CO2 (g)
E) All of the above are calcination processes.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
75
What is the name and the formula of the most useful ore of aluminum?

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
76
arises when the unpaired electrons of the atoms or ions in a solid are influenced by the orientations of the electrons of their neighbors.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
77
In the Bayer process, the purpose of filtration after the ore has been digested in concentrated sodium hydroxide is .
A) to remove the sodium ions from the sodium hydroxide solution
B) to separate the soluble aluminum complex from the insoluble iron impurities
C) to remove the anode sludge
D) to separate the insoluble aluminum oxide from the soluble iron impurity
E) to separate the aluminum metal from the hydroxide ions
A) to remove the sodium ions from the sodium hydroxide solution
B) to separate the soluble aluminum complex from the insoluble iron impurities
C) to remove the anode sludge
D) to separate the insoluble aluminum oxide from the soluble iron impurity
E) to separate the aluminum metal from the hydroxide ions

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
78
Most transition metal ions contain partially occupied subshells.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
79
An aqueous solution of Fe(NO3)3 will slowly form a red- brown precipitate due to the formation of
)
A) Fe(OH)3
B) FeO
C) Fe3O4
D) FeO2
E) Fe(OH)2
)
A) Fe(OH)3
B) FeO
C) Fe3O4
D) FeO2
E) Fe(OH)2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
80
Metallurgical processes that utilize high temperatures are collectively called
A) alloying.
B) pyrometallurgy.
C) roasting.
D) electrometallurgy.
E) hydrometallurgy.
A) alloying.
B) pyrometallurgy.
C) roasting.
D) electrometallurgy.
E) hydrometallurgy.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck


