Exam 23: Metals and Metallurgy
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Why is either pure oxygen or oxygen diluted with argon used in a converter instead of air?
Free
(Multiple Choice)
4.7/5  (28)
(28)
Correct Answer:
C
In the Bayer process, the purpose of filtration after the ore has been digested in concentrated sodium hydroxide is .
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
B
A basic slag is needed in steelmaking to
Free
(Multiple Choice)
4.9/5  (36)
(36)
Correct Answer:
E
What is the purpose of adding zinc powder to a solution of Au(CN)2- ?
(Multiple Choice)
5.0/5  (31)
(31)
Part of the Bayer process involves the digestion of crushed ore in concentrated aqueous sodium hydroxide. This process carried out at high pressure .
(Multiple Choice)
4.9/5  (40)
(40)
Roasting HgS in the presence of oxygen produces the free metal and SO2. What is the coefficient of HgS when the equation for this reaction is completed and balanced?
(Multiple Choice)
4.7/5  (26)
(26)
CuS has a very low solubility in water. However, it will dissolve in nitric acid due to the following reaction. 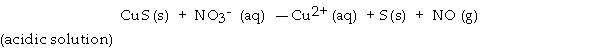 What is the coefficient of CuS when this equation is balanced?
What is the coefficient of CuS when this equation is balanced?
(Multiple Choice)
4.8/5  (35)
(35)
The undesirable material that is separated from an ore during the concentration process is called
)
(Multiple Choice)
4.8/5  (40)
(40)
Processes used to reduce metal ores or to refine metals that are based on the process of electrolysis are collectively referred to as _.
(Multiple Choice)
4.9/5  (46)
(46)
Selectively dissolving a metal- containing compound from an ore is called
(Multiple Choice)
4.9/5  (39)
(39)
Roasting of the disulfide of molybdenum in O2 produces which products?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following are not commonly used as sources of metals?
(Multiple Choice)
4.9/5  (36)
(36)
Which one of the following metallic elements is most likely to be found as the free metal in nature?
(Multiple Choice)
4.7/5  (43)
(43)
Showing 1 - 20 of 112
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)