Deck 20: Electrochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/90
Play
Full screen (f)
Deck 20: Electrochemistry
1
Which of the following reactions will occur spontaneously as written?
A) 3Fe (s) + 2Cr3+ (aq) -2Cr (s) + 3Fe2+ (aq)
B) 3Sn4+ (aq) + 2Cr (s) -2Cr3+ (aq) + 3Sn2+ (aq)
C) Sn4+ (aq) + Fe2+ (aq) -Sn2+ (aq) + Fe (s)
D) 3Fe2+ (aq) -Fe (s) + 2Fe3+ (aq)
E) Sn4+ (aq) + Fe3+ (aq) -Sn2+ (aq) + Fe2+ (aq)
A) 3Fe (s) + 2Cr3+ (aq) -2Cr (s) + 3Fe2+ (aq)
B) 3Sn4+ (aq) + 2Cr (s) -2Cr3+ (aq) + 3Sn2+ (aq)
C) Sn4+ (aq) + Fe2+ (aq) -Sn2+ (aq) + Fe (s)
D) 3Fe2+ (aq) -Fe (s) + 2Fe3+ (aq)
E) Sn4+ (aq) + Fe3+ (aq) -Sn2+ (aq) + Fe2+ (aq)
3Sn4+ (aq) + 2Cr (s) -2Cr3+ (aq) + 3Sn2+ (aq)
2
Consider an electrochemical cell based on the reaction:

Which of the following actions would not change the measured cell potential?
A) lowering the pH in the cathode compartment
B) increasing the pressure of hydrogen gas in the cathode compartment
C) addition of more tin metal to the anode compartment
D) increasing the tin (II) ion concentration in the anode compartment
E) Any of the above will change the measured cell potential.

Which of the following actions would not change the measured cell potential?
A) lowering the pH in the cathode compartment
B) increasing the pressure of hydrogen gas in the cathode compartment
C) addition of more tin metal to the anode compartment
D) increasing the tin (II) ion concentration in the anode compartment
E) Any of the above will change the measured cell potential.
addition of more tin metal to the anode compartment
3
Using Table 20.1, which substance can be oxidized by O2 (g) in acidic aqueous solution?
A) Ni2+ (aq)
B) Br- (aq)
C) Cu2+ (aq)
D) Ag (s)
E) Br2 (l)
A) Ni2+ (aq)
B) Br- (aq)
C) Cu2+ (aq)
D) Ag (s)
E) Br2 (l)
Br2 (l)
4
Which of the following reactions is a redox reaction?
a. K2CrO4 + BaCl2 - BaCrO4 + 2KCl
b. Pb22+ + 2Br- - PbBr
c. Cu + S - CuS
A) (a) only
B) (b) only
C) (c) only
D) (a) and (c)
E) (b) and (c)
a. K2CrO4 + BaCl2 - BaCrO4 + 2KCl
b. Pb22+ + 2Br- - PbBr
c. Cu + S - CuS
A) (a) only
B) (b) only
C) (c) only
D) (a) and (c)
E) (b) and (c)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the halogens in Table 20.1 is the strongest oxidizing agent?
A) F2
B) Cl2
C) Br2
D) I2
E) All of the halogens have equal strength as oxidizing agents.
A) F2
B) Cl2
C) Br2
D) I2
E) All of the halogens have equal strength as oxidizing agents.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
6
Which transformation could take place at the anode of an electrochemical cell?
A) CO2 -C2O42-
B) O2 -H2O2
C) VO2+ - VO2+
D) NO - NO3-
E) H2AsO4 -H3AsO3
A) CO2 -C2O42-
B) O2 -H2O2
C) VO2+ - VO2+
D) NO - NO3-
E) H2AsO4 -H3AsO3

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
7
Which transformation could take place at the cathode of an electrochemical cell?
A) Br2 - BrO3-
B) NO - HNO2
C) MnO2 - MnO4-
D) HSO4- -H2SO3
E) Mn2+ - MnO4-
A) Br2 - BrO3-
B) NO - HNO2
C) MnO2 - MnO4-
D) HSO4- -H2SO3
E) Mn2+ - MnO4-

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
8
The standard cell potential (E°) of a voltaic cell constructed using the cell reaction below is 0.76 V:
![<strong>The standard cell potential (E°) of a voltaic cell constructed using the cell reaction below is 0.76 V: With P<sub>H</sub><sub>2 </sub>= 1.0 atm and [Zn<sup>2</sup><sup>+</sup>] = 1.0 M, the cell potential is 0.66 V. The concentration of H<sup>+ </sup>in the Cathode compartment is _ M.</strong> A) 4.2 × 10<sup>-</sup><sup> </sup><sup>4</sup> B) 1.0 × 10<sup>- </sup><sup>12</sup> C) 4.9 × 10<sup>1</sup> D) 2.0 × 10<sup>-</sup><sup> </sup><sup>2</sup> E) 1.4 × 10<sup>-</sup><sup> </sup><sup>1</sup>](https://storage.examlex.com/TB1819/11ead62c_1f1e_35f4_ac95_0934b47cea8a_TB1819_00.jpg)
With PH2 = 1.0 atm and [Zn2+] = 1.0 M, the cell potential is 0.66 V. The concentration of H+ in the
Cathode compartment is _ M.
A) 4.2 × 10- 4
B) 1.0 × 10- 12
C) 4.9 × 101
D) 2.0 × 10- 2
E) 1.4 × 10- 1
![<strong>The standard cell potential (E°) of a voltaic cell constructed using the cell reaction below is 0.76 V: With P<sub>H</sub><sub>2 </sub>= 1.0 atm and [Zn<sup>2</sup><sup>+</sup>] = 1.0 M, the cell potential is 0.66 V. The concentration of H<sup>+ </sup>in the Cathode compartment is _ M.</strong> A) 4.2 × 10<sup>-</sup><sup> </sup><sup>4</sup> B) 1.0 × 10<sup>- </sup><sup>12</sup> C) 4.9 × 10<sup>1</sup> D) 2.0 × 10<sup>-</sup><sup> </sup><sup>2</sup> E) 1.4 × 10<sup>-</sup><sup> </sup><sup>1</sup>](https://storage.examlex.com/TB1819/11ead62c_1f1e_35f4_ac95_0934b47cea8a_TB1819_00.jpg)
With PH2 = 1.0 atm and [Zn2+] = 1.0 M, the cell potential is 0.66 V. The concentration of H+ in the
Cathode compartment is _ M.
A) 4.2 × 10- 4
B) 1.0 × 10- 12
C) 4.9 × 101
D) 2.0 × 10- 2
E) 1.4 × 10- 1

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
9
Which element is oxidized in the reaction below? 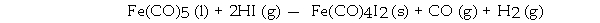
A) I
B) H
C) O
D) C
E) Fe
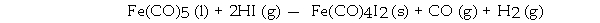
A) I
B) H
C) O
D) C
E) Fe

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
10
Cathodic protection of a metal pipe against corrosion usually entails
A) attaching an active metal to make the pipe the anode in an electrochemical cell.
B) coating the pipe with a fluoropolymer to act as a source of fluoride ion (since the latter is so hard to oxidize)
C) coating the pipe with another metal whose standard reduction potential is less negative than that of the pipe.
D) attaching a dry cell to reduce any metal ions which might be formed.
E) attaching an active metal to make the pipe the cathode in an electrochemical cell.
A) attaching an active metal to make the pipe the anode in an electrochemical cell.
B) coating the pipe with a fluoropolymer to act as a source of fluoride ion (since the latter is so hard to oxidize)
C) coating the pipe with another metal whose standard reduction potential is less negative than that of the pipe.
D) attaching a dry cell to reduce any metal ions which might be formed.
E) attaching an active metal to make the pipe the cathode in an electrochemical cell.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
11
What is the anode in an alkaline battery ?
A) Mn2O3
B) MnO2
C) KOH
D) Pt
E) Zn powder
A) Mn2O3
B) MnO2
C) KOH
D) Pt
E) Zn powder

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
12
The electrolysis of molten AlCl3 for 3.25 hr with an electrical current of 15.0 A produces
G of aluminum metal.
A) 16.4
B) 4.55 × 10- 3
C) 147
D) 0.606
E) 49.1
G of aluminum metal.
A) 16.4
B) 4.55 × 10- 3
C) 147
D) 0.606
E) 49.1

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following is the best oxidizing agent ?
A) O2
B) Na
C) H2
D) Ca
E) Li
A) O2
B) Na
C) H2
D) Ca
E) Li

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
14
Consider an electrochemical cell based on the reaction:
![<strong>Consider an electrochemical cell based on the reaction: Which of the following actions would change the measured cell potential?</strong> A) lowering the pH in the cathode compartment B) increasing the pressure of hydrogen gas in the cathode compartment C) increasing the pH in the cathode compartment D) increasing the [Sn<sup>2</sup><sup>+</sup>] in the anode compartment E) Any of the above will change the measure cell potential.](https://storage.examlex.com/TB1819/11ead62c_1f1b_c4ef_ac95_0f382923d75f_TB1819_00.jpg)
Which of the following actions would change the measured cell potential?
A) lowering the pH in the cathode compartment
B) increasing the pressure of hydrogen gas in the cathode compartment
C) increasing the pH in the cathode compartment
D) increasing the [Sn2+] in the anode compartment
E) Any of the above will change the measure cell potential.
![<strong>Consider an electrochemical cell based on the reaction: Which of the following actions would change the measured cell potential?</strong> A) lowering the pH in the cathode compartment B) increasing the pressure of hydrogen gas in the cathode compartment C) increasing the pH in the cathode compartment D) increasing the [Sn<sup>2</sup><sup>+</sup>] in the anode compartment E) Any of the above will change the measure cell potential.](https://storage.examlex.com/TB1819/11ead62c_1f1b_c4ef_ac95_0f382923d75f_TB1819_00.jpg)
Which of the following actions would change the measured cell potential?
A) lowering the pH in the cathode compartment
B) increasing the pressure of hydrogen gas in the cathode compartment
C) increasing the pH in the cathode compartment
D) increasing the [Sn2+] in the anode compartment
E) Any of the above will change the measure cell potential.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
15
Which transformation could take place at the anode of an electrochemical cell?
A) O2 to H2O
B) Cr2O72- -Cr2+
C) HAsO2 to As
D) F2 toF-
E) None of the above could take place at the anode.
A) O2 to H2O
B) Cr2O72- -Cr2+
C) HAsO2 to As
D) F2 toF-
E) None of the above could take place at the anode.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
16
One of the differences between a voltaic cell and an electrolytic cell is that in an electrolytic cell
)
A) oxidation occurs at the cathode
B) an electric current is produced by a chemical reaction
C) O2 gas is produced at the cathode
D) electrons flow toward the anode
E) a nonspontaneous reaction is forced to occur
)
A) oxidation occurs at the cathode
B) an electric current is produced by a chemical reaction
C) O2 gas is produced at the cathode
D) electrons flow toward the anode
E) a nonspontaneous reaction is forced to occur

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
17
Which substance is the reducing agent in the following reaction? 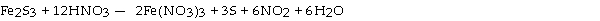
A) NO2
B) Fe2S3
C) H2O
D) S
E) HNO3
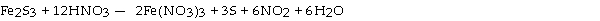
A) NO2
B) Fe2S3
C) H2O
D) S
E) HNO3

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
18
A voltaic cell is constructed with two Zn2+- Zn electrodes, where the half- reaction is  The concentrations of zinc ion in the two compartments are 5.50 M and 1.11 × 10- 2 M, respectively.
The concentrations of zinc ion in the two compartments are 5.50 M and 1.11 × 10- 2 M, respectively.
The cell emf is V.
A) - 378
B) - 0.761
C) 0.160
D) - 1.54 × 10- 3
E) 0.0798
 The concentrations of zinc ion in the two compartments are 5.50 M and 1.11 × 10- 2 M, respectively.
The concentrations of zinc ion in the two compartments are 5.50 M and 1.11 × 10- 2 M, respectively.The cell emf is V.
A) - 378
B) - 0.761
C) 0.160
D) - 1.54 × 10- 3
E) 0.0798

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
19
The most useful ore of aluminum is bauxite, in which Al is present as hydrated oxides,
Al2O3 ÷ H2O. The number of kilowatt- hours of electricity required to produce 4.00 kg of aluminum from electrolysis of compounds from bauxite is when the applied emf is
5)00 V.
A) 59.6
B) 0.0168
C) 39.7
D) 19.9
E) 0.0596
Al2O3 ÷ H2O. The number of kilowatt- hours of electricity required to produce 4.00 kg of aluminum from electrolysis of compounds from bauxite is when the applied emf is
5)00 V.
A) 59.6
B) 0.0168
C) 39.7
D) 19.9
E) 0.0596

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following reactions will occur spontaneously as written?
A) 2Cr (s) + 3Fe2+ (s) - 3Fe (s) + 2Cr3+ (aq)
B) Sn4+ (aq) + Fe2+ (s) - Sn2+ (aq) + Fe (s)
C) 3Fe2+ (aq) + Cr3+ (aq) - Cr (s) + 3Fe3+ (aq)
D) Sn2+ (aq) + Fe2+ (s) -Sn4+ (aq) + Fe3+ (aq)
E) 2Cr3+ (aq) + 3Sn2+ (aq) - 3Sn4+ (aq) + 2Cr (s)
A) 2Cr (s) + 3Fe2+ (s) - 3Fe (s) + 2Cr3+ (aq)
B) Sn4+ (aq) + Fe2+ (s) - Sn2+ (aq) + Fe (s)
C) 3Fe2+ (aq) + Cr3+ (aq) - Cr (s) + 3Fe3+ (aq)
D) Sn2+ (aq) + Fe2+ (s) -Sn4+ (aq) + Fe3+ (aq)
E) 2Cr3+ (aq) + 3Sn2+ (aq) - 3Sn4+ (aq) + 2Cr (s)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
21
A voltaic cell is constructed with two silver- silver chloride electrodes, where the half- reaction is  The concentrations of chloride ion in the two compartments are 0.0222 M and 2.22 M, respectively.
The concentrations of chloride ion in the two compartments are 0.0222 M and 2.22 M, respectively.
The cell emf is V.
A) 22.2
B) 0.232
C) 0.118
D) 0.00222
E) 0.212
 The concentrations of chloride ion in the two compartments are 0.0222 M and 2.22 M, respectively.
The concentrations of chloride ion in the two compartments are 0.0222 M and 2.22 M, respectively.The cell emf is V.
A) 22.2
B) 0.232
C) 0.118
D) 0.00222
E) 0.212

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
22
In a lead- acid battery, the electrodes are consumed. In this battery,
A) the anode is PbSO4.
B) the cathode is PbSO4.
C) the anode is PbO2.
D) the cathode is Pb.
E) the anode is Pb.
A) the anode is PbSO4.
B) the cathode is PbSO4.
C) the anode is PbO2.
D) the cathode is Pb.
E) the anode is Pb.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
23
Which one of the following reactions is a redox reaction?
A) H2O + NaCl - NaOH + HCl
B) NaOH + HCl - NaCl + H2O
C) Pb2+ + 2Cl- - PbCl2
D) AgNO3 + HCl - HNO3 + AgCl
E) None of the above is a redox reaction.
A) H2O + NaCl - NaOH + HCl
B) NaOH + HCl - NaCl + H2O
C) Pb2+ + 2Cl- - PbCl2
D) AgNO3 + HCl - HNO3 + AgCl
E) None of the above is a redox reaction.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the number of grams of aluminum produced in 30 minutes by electrolysis of AlCl3 at a current of 12 A.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
25
What is the coefficient of the permanganate ion when the following equation is balanced? 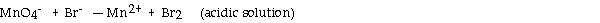
A) 2
B) 1
C) 5
D) 3
E) 4
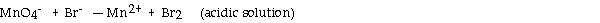
A) 2
B) 1
C) 5
D) 3
E) 4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
26
How many seconds are required to produce 4.00 g of aluminum metal from the electrolysis of molten AlCl3 with an electrical current of 12.0 A?
A) 27.0
B) 3.57 × 103
C) 1.19 × 103
D) 9.00
E) 2.90 × 105
A) 27.0
B) 3.57 × 103
C) 1.19 × 103
D) 9.00
E) 2.90 × 105

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
27
The town of Natrium, West Virginia, derives its name from the sodium produced in the electrolysis of molten sodium chloride (NaCl) mined from ancient salt deposits. The number of kilowatt- hours of electricity required to produce 4.60 kg of metallic sodium from the electrolysis of molten NaCl(s) is when the applied emf is 4.50 V.
A) 48.3
B) 12.1
C) 0.0241
D) 0.0414
E) 24.1
A) 48.3
B) 12.1
C) 0.0241
D) 0.0414
E) 24.1

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
28
How many kilowatt- hours of electricity are used to produce 3.00 kg of magnesium in the electrolysis of molten MgCl2 with an applied emf of 4.50 V?
A) 7.4
B) 14.9
C) 0.0336
D) 0.0298
E) 29.8
A) 7.4
B) 14.9
C) 0.0336
D) 0.0298
E) 29.8

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
29
The standard emf for the cell using the overall cell reaction below is +2.20 V:
![<strong>The standard emf for the cell using the overall cell reaction below is +2.20 V: The emf generated by the cell when [Al<sup>3</sup><sup>+</sup>] = 4.5 × 10<sup>-</sup><sup> </sup><sup>3</sup><sup> </sup>M and [I<sup>-</sup><sup> </sup>] = 0.15 M is V.</strong> A) 2.30 B) 2.23 C) 2.39 D) 2.10 E) 2.20](https://storage.examlex.com/TB1819/11ead62c_1f1f_e3a9_ac95_c99e28eb68b5_TB1819_00.jpg)
The emf generated by the cell when [Al3+] = 4.5 × 10- 3 M and [I- ] = 0.15 M is V.
A) 2.30
B) 2.23
C) 2.39
D) 2.10
E) 2.20
![<strong>The standard emf for the cell using the overall cell reaction below is +2.20 V: The emf generated by the cell when [Al<sup>3</sup><sup>+</sup>] = 4.5 × 10<sup>-</sup><sup> </sup><sup>3</sup><sup> </sup>M and [I<sup>-</sup><sup> </sup>] = 0.15 M is V.</strong> A) 2.30 B) 2.23 C) 2.39 D) 2.10 E) 2.20](https://storage.examlex.com/TB1819/11ead62c_1f1f_e3a9_ac95_c99e28eb68b5_TB1819_00.jpg)
The emf generated by the cell when [Al3+] = 4.5 × 10- 3 M and [I- ] = 0.15 M is V.
A) 2.30
B) 2.23
C) 2.39
D) 2.10
E) 2.20

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
30
The dependence of cell emf on concentration is expressed in the .

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
31
What is the coefficient of Fe3+ when the following equation is balanced? 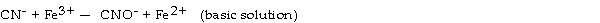
A) 1
B) 2
C) 3
D) 4
E) 5
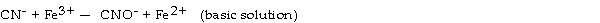
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
32
The potential (E) to move K+ from the extracellular fluid to the intracellular fluid necessitates work. The sign for this potential is _ _.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
33
Which one of the following types of elements is most likely to be a good oxidizing agent?
A) alkali metals
B) transition elements
C) halogens
D) alkaline earth elements
E) lanthanides
A) alkali metals
B) transition elements
C) halogens
D) alkaline earth elements
E) lanthanides

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
34
In the formula ΔG = - nFE , F is the _.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
35
The purpose of the salt bridge in an electrochemical cell is to .
A) provide a source of ions to react at the anode and cathode.
B) maintain electrical neutrality in the half- cells via migration of ions.
C) provide a means for electrons to travel from the anode to the cathode.
D) provide a means for electrons to travel from the cathode to the anode.
E) provide oxygen to facilitate oxidation at the anode.
A) provide a source of ions to react at the anode and cathode.
B) maintain electrical neutrality in the half- cells via migration of ions.
C) provide a means for electrons to travel from the anode to the cathode.
D) provide a means for electrons to travel from the cathode to the anode.
E) provide oxygen to facilitate oxidation at the anode.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
36
The major product of a hydrogen fuel cell is .

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
37
The most difficult species to reduce and the poorest oxidizing agent is _ .

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
38
Using Table 20.1, which substance can oxidize I- (aq) to I2 (s) ?
A) Ag (s)
B) Br2 (l)
C) Cu2+ (aq)
D) Ni2+ (aq)
E) Br- (aq)
A) Ag (s)
B) Br2 (l)
C) Cu2+ (aq)
D) Ni2+ (aq)
E) Br- (aq)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
39
The standard emf for the cell using the overall cell reaction below is +0.48 V:
![<strong>The standard emf for the cell using the overall cell reaction below is +0.48 V: The emf generated by the cell when [Ni<sup>2</sup><sup>+</sup>] = 2.50 M and [Zn<sup>2</sup><sup>+</sup>] = 0.100 M is V.</strong> A) 0.40 B) 0.50 C) 0.52 D) 0.44 E) 0.56](https://storage.examlex.com/TB1819/11ead62c_1f1f_9588_ac95_77b6977504b8_TB1819_00.jpg)
The emf generated by the cell when [Ni2+] = 2.50 M and [Zn2+] = 0.100 M is V.
A) 0.40
B) 0.50
C) 0.52
D) 0.44
E) 0.56
![<strong>The standard emf for the cell using the overall cell reaction below is +0.48 V: The emf generated by the cell when [Ni<sup>2</sup><sup>+</sup>] = 2.50 M and [Zn<sup>2</sup><sup>+</sup>] = 0.100 M is V.</strong> A) 0.40 B) 0.50 C) 0.52 D) 0.44 E) 0.56](https://storage.examlex.com/TB1819/11ead62c_1f1f_9588_ac95_77b6977504b8_TB1819_00.jpg)
The emf generated by the cell when [Ni2+] = 2.50 M and [Zn2+] = 0.100 M is V.
A) 0.40
B) 0.50
C) 0.52
D) 0.44
E) 0.56

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
40
The quantity of charge passing a point in a circuit in one second when the current is one ampere is called a .

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
41
The more the value of E°red, the greater the driving force for reduction.
A) extensive
B) positive
C) negative
D) endothermic
E) exothermic
A) extensive
B) positive
C) negative
D) endothermic
E) exothermic

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
42
How many minutes will it take to plate out 2.19 g of chromium metal from a solution of Cr3+ using a current of 35.2 amps in an electrolyte cell ?
A) 17.3
B) 1.92
C) 5.77
D) 346
E) 115
A) 17.3
B) 1.92
C) 5.77
D) 346
E) 115

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
43
The half- reaction occurring at the anode in the balanced reaction shown below is . 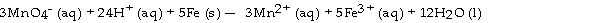
A) 2MnO4- (aq) + 12H+ (aq) + 6e- - 2Mn2+ (aq) + 3H2O (l)
B) Fe (s) -Fe2+ (aq) + 2e-
C) Fe (s) -Fe3+ (aq) + 3e-
D) Fe2+ (aq) - Fe3+ (aq) + e-
E) MnO4- (aq) + 8H+ (aq) + 5e- -Mn2+ (aq) + 4H2O (l)
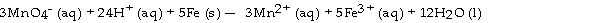
A) 2MnO4- (aq) + 12H+ (aq) + 6e- - 2Mn2+ (aq) + 3H2O (l)
B) Fe (s) -Fe2+ (aq) + 2e-
C) Fe (s) -Fe3+ (aq) + 3e-
D) Fe2+ (aq) - Fe3+ (aq) + e-
E) MnO4- (aq) + 8H+ (aq) + 5e- -Mn2+ (aq) + 4H2O (l)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
44
What is the oxidation number of manganese in the Mn O 1- ion?
A) +7
B) +5
C) +4
D) +1
E) +2
A) +7
B) +5
C) +4
D) +1
E) +2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
45
Corrosion of iron is retarded by .
A) high pH conditions
B) low pH conditions
C) the presence of salts
D) both the presence of salts and high pH conditions
E) both the presence of salts and low pH conditions
A) high pH conditions
B) low pH conditions
C) the presence of salts
D) both the presence of salts and high pH conditions
E) both the presence of salts and low pH conditions

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
46
How many seconds are required to produce 1.0 g of silver metal by the electrolysis of a AgNO3 solution using a current of 30 amps _ ?
A) 3.7 × 10- 5
B) 3.2 × 103
C) 2.7 × 104
D) 60
E) 30
A) 3.7 × 10- 5
B) 3.2 × 103
C) 2.7 × 104
D) 60
E) 30

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
47
The standard cell potential (E°cell) for the voltaic cell based on the reaction below is V. Sn2+ (aq) + 2Fe3+ (aq) - 2Fe2+ (aq) + Sn4+ (aq)
A) +1.39
B) +1.21
C) - 0.46
D) +0.46
E) +0.617
A) +1.39
B) +1.21
C) - 0.46
D) +0.46
E) +0.617

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
48
The balanced half- reaction in which dichromate ion is reduced to chromium(III) ion is a
Process.
A) four- electron
B) six- electron
C) three- electron
D) two- electron
E) twelve- electron
Process.
A) four- electron
B) six- electron
C) three- electron
D) two- electron
E) twelve- electron

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
49
Galvanized iron is iron coated with .
A) phosphate.
B) iron oxide.
C) chromium.
D) zinc.
E) magnesium.
A) phosphate.
B) iron oxide.
C) chromium.
D) zinc.
E) magnesium.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
50
What current (in a) is required to plate out 1.22 g of nickel from a solution of Ni2+ in 1.0 hour __________ ?
A) 12.9
B) 1.11
C) 4.01 × 103
D) 2.34
E) 65.4
A) 12.9
B) 1.11
C) 4.01 × 103
D) 2.34
E) 65.4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
51
The balanced half- reaction in which sulfate ion is reduced to sulfite ion is a process.
A) three- electron
B) one- electron
C) six- electron
D) two- electron
E) four- electron
A) three- electron
B) one- electron
C) six- electron
D) two- electron
E) four- electron

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
52
A voltaic cell can be constructed of the same species as long as the are different.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
53
1V = .
A) 1 C/J
B) 1 J/s
C) 1 J/C
D) 96485 C
E) 1 amp · s
A) 1 C/J
B) 1 J/s
C) 1 J/C
D) 96485 C
E) 1 amp · s

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
54
The standard cell potential (E°cell) for the voltaic cell based on the reaction below is V. 
A) +1.57
B) - 1.45
C) +3.05
D) +2.99
E) +1.51

A) +1.57
B) - 1.45
C) +3.05
D) +2.99
E) +1.51

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
55
The standard cell potential (E°cell) of the reaction below is +0.126 V. The value of OG° for the reaction is kJ/mol.
Pb (s) + 2H+ (aq) -Pb2+ (aq) + H2 (g)
A) - 24
B) +24
C) - 12
D) +12
E) - 50
Pb (s) + 2H+ (aq) -Pb2+ (aq) + H2 (g)
A) - 24
B) +24
C) - 12
D) +12
E) - 50

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
56
is the oxidizing agent in the reaction below. 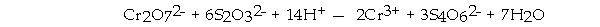
A) S4O62-
B) Cr2O72-
C) H+
D) Cr3+
E) S2O32-
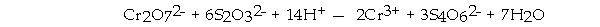
A) S4O62-
B) Cr2O72-
C) H+
D) Cr3+
E) S2O32-

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
57
The anode of the alkaline battery is powdered zinc in a gel that contacts .

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
58
At constant temperature and pressure the Gibbs free energy value is a measure of the
of a process.
of a process.

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
59
When iron is coated with a thin layer of zinc to protect against corrosion, the iron is said to be .

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
60
The standard cell potential (E°cell) for the voltaic cell based on the reaction below is V. 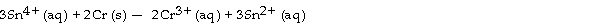
A) +0.89
B) - 0.59
C) - 1.02
D) +1.94
E) +2.53
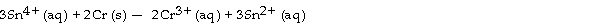
A) +0.89
B) - 0.59
C) - 1.02
D) +1.94
E) +2.53

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
61
Which substance is serving as the reducing agent in the following reaction? 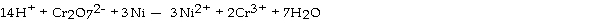
A) Ni2+
B) Cr2O72-
C) Ni
D) H+
E) H2O
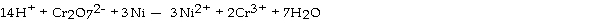
A) Ni2+
B) Cr2O72-
C) Ni
D) H+
E) H2O

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
62
electrons appear in the following half- reaction when it is balanced. 
A) 4
B) 1
C) 2
D) 3
E) 6

A) 4
B) 1
C) 2
D) 3
E) 6

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
63
The electrode at which oxidation occurs is called the .
A) oxidizing agent
B) voltaic cell
C) anode
D) reducing agent
E) cathode
A) oxidizing agent
B) voltaic cell
C) anode
D) reducing agent
E) cathode

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
64
Which substance is the reducing agent in the reaction below? 
A) PbSO4
B) Pb
C) H2SO4
D) PbO2
E) H2O

A) PbSO4
B) Pb
C) H2SO4
D) PbO2
E) H2O

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
65
How many grams of copper will be plated out by a current of 2.3 A applied for 25 minutes to a 0.50- M solution of copper(II) sulfate _ ?
A) 0.019
B) 1.8 × 10- 2
C) 1.1
D) 2.2
E) 0.036
A) 0.019
B) 1.8 × 10- 2
C) 1.1
D) 2.2
E) 0.036

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
66
is reduced in the following reaction:
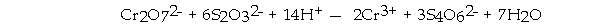
A) Cr2O72-
B) S4O62-
C) H+
D) S2O32-
E) Cr3+
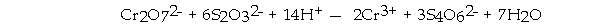
A) Cr2O72-
B) S4O62-
C) H+
D) S2O32-
E) Cr3+

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
67
The lead- containing reactant(s) consumed during recharging of a lead- acid battery is/are
)
A) PbO2 (s) only
B) PbSO4 (s) only
C) Pb (s) only
D) both PbO2 (s) and PbSO4 (s)
E) both Pb (s) and PbO2 (s)
)
A) PbO2 (s) only
B) PbSO4 (s) only
C) Pb (s) only
D) both PbO2 (s) and PbSO4 (s)
E) both Pb (s) and PbO2 (s)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
68
How many grams of CuS are obtained by passing a current of 12 A through a solution of CuSO4 for 15 minutes ?
A) 7.1
B) 14
C) 1.8
D) 3.6
E) 0.016
A) 7.1
B) 14
C) 1.8
D) 3.6
E) 0.016

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
69
The standard cell potential (E°cell) for the reaction below is +0.63 V. The cell potential for this reaction is V when [ Zn2+] = 1.0 M and [Pb2+] = 2.0 × 10- 4 M. ![<strong>The standard cell potential (E°<sub>cell</sub>) for the reaction below is +0.63 V. The cell potential for this reaction is V when [ Zn<sup>2</sup><sup>+</sup>] = 1.0 M and [Pb<sup>2</sup><sup>+</sup>] = 2.0 × 10<sup>-</sup><sup> </sup><sup>4</sup><sup> </sup>M. </strong> A) 0.74 B) 0.85 C) 0.52 D) 0.63 E) 0.41](https://storage.examlex.com/TB1819/11ead62c_1f22_c9e2_ac95_01bf5f62ff66_TB1819_00.jpg)
A) 0.74
B) 0.85
C) 0.52
D) 0.63
E) 0.41
![<strong>The standard cell potential (E°<sub>cell</sub>) for the reaction below is +0.63 V. The cell potential for this reaction is V when [ Zn<sup>2</sup><sup>+</sup>] = 1.0 M and [Pb<sup>2</sup><sup>+</sup>] = 2.0 × 10<sup>-</sup><sup> </sup><sup>4</sup><sup> </sup>M. </strong> A) 0.74 B) 0.85 C) 0.52 D) 0.63 E) 0.41](https://storage.examlex.com/TB1819/11ead62c_1f22_c9e2_ac95_01bf5f62ff66_TB1819_00.jpg)
A) 0.74
B) 0.85
C) 0.52
D) 0.63
E) 0.41

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
70
How many grams of Ca metal are produced by the electrolysis of molten CaBr2 using a current of
30)0 amp for 10.0 hours ?
A) 448
B) 0.0622
C) 112
D) 224
E) 22.4
30)0 amp for 10.0 hours ?
A) 448
B) 0.0622
C) 112
D) 224
E) 22.4

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
71
The standard cell potential (E°cell) for the voltaic cell based on the reaction below is V. 
A) +0.30
B) +3.10
C) +2.80
D) - 0.16
E) +0.83

A) +0.30
B) +3.10
C) +2.80
D) - 0.16
E) +0.83

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
72
The gain of electrons by an element is called .
A) oxidation
B) reduction
C) disproportionation
D) sublimation
E) fractionation
A) oxidation
B) reduction
C) disproportionation
D) sublimation
E) fractionation

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
73
The reduction half reaction occurring in the standard hydrogen electrode is _.
A) 2H+ (aq, 1M) + 2e- -H2 (g, 1 atm)
B) 2H+ (aq, 1M) + Cl2 (aq) - 2HCl (aq)
C) H2 (g, 1 atm) - 2H+ (aq, 1M) + 2e-
D) 2H+ (aq) + 2OH- -H2O (l)
E) O2 (g) + 4H+ (aq) + 4e- - 2H2O (l)
A) 2H+ (aq, 1M) + 2e- -H2 (g, 1 atm)
B) 2H+ (aq, 1M) + Cl2 (aq) - 2HCl (aq)
C) H2 (g, 1 atm) - 2H+ (aq, 1M) + 2e-
D) 2H+ (aq) + 2OH- -H2O (l)
E) O2 (g) + 4H+ (aq) + 4e- - 2H2O (l)

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
74
What is the oxidation number of potassium in KMnO4?
A) +2
B) +1
C) - 1
D) +3
E) 0
A) +2
B) +1
C) - 1
D) +3
E) 0

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
75
What is the oxidation number of manganese in MnO2?
A) +1
B) +3
C) +4
D) +7
E) +2
A) +1
B) +3
C) +4
D) +7
E) +2

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
76
The balanced half- reaction in which chlorine gas is reduced to the aqueous chloride ion is a
Process.
A) three- electron
B) four- electron
C) two- electron
D) six- electron
E) one- electron
Process.
A) three- electron
B) four- electron
C) two- electron
D) six- electron
E) one- electron

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
77
The balanced half- reaction in which dichromate ion is reduced to chromium metal is a process.
A) two- electron
B) four- electron
C) three- electron
D) six- electron
E) twelve- electron
A) two- electron
B) four- electron
C) three- electron
D) six- electron
E) twelve- electron

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
78
In a voltaic cell, electrons flow from the to the .
A) anode, salt bridge
B) salt bride, anode
C) anode, cathode
D) salt bridge, cathode
E) cathode, anode
A) anode, salt bridge
B) salt bride, anode
C) anode, cathode
D) salt bridge, cathode
E) cathode, anode

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
79
The standard cell potential (E°cell) for the reaction below is +1.10 V. The cell potential for this reaction is V when the concentration of [Cu2+] = 1.0 × 10- 5 M and [Zn2+] = 1.0 M. ![<strong>The standard cell potential (E°<sub>cell</sub>) for the reaction below is +1.10 V. The cell potential for this reaction is V when the concentration of [Cu<sup>2</sup><sup>+</sup>] = 1.0 × 10<sup>-</sup><sup> </sup><sup>5</sup><sup> </sup>M and [Zn<sup>2</sup><sup>+</sup>] = 1.0 M. </strong> A) 1.25 B) 0.95 C) 1.10 D) 0.80 E) 1.40](https://storage.examlex.com/TB1819/11ead62c_1f23_1803_ac95_f9aa52127bd4_TB1819_00.jpg)
A) 1.25
B) 0.95
C) 1.10
D) 0.80
E) 1.40
![<strong>The standard cell potential (E°<sub>cell</sub>) for the reaction below is +1.10 V. The cell potential for this reaction is V when the concentration of [Cu<sup>2</sup><sup>+</sup>] = 1.0 × 10<sup>-</sup><sup> </sup><sup>5</sup><sup> </sup>M and [Zn<sup>2</sup><sup>+</sup>] = 1.0 M. </strong> A) 1.25 B) 0.95 C) 1.10 D) 0.80 E) 1.40](https://storage.examlex.com/TB1819/11ead62c_1f23_1803_ac95_f9aa52127bd4_TB1819_00.jpg)
A) 1.25
B) 0.95
C) 1.10
D) 0.80
E) 1.40

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck
80
The relationship between the change in Gibbs free energy and the emf of an electrochemical cell is given by .
A) ΔG = - nF
ERT
B) ΔG = - nF
E
C) ΔG = - nFE
D) ΔG = - nRTF
E) ΔG = - E
NF
A) ΔG = - nF
ERT
B) ΔG = - nF
E
C) ΔG = - nFE
D) ΔG = - nRTF
E) ΔG = - E
NF

Unlock Deck
Unlock for access to all 90 flashcards in this deck.
Unlock Deck
k this deck


