Exam 20: Electrochemistry
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
In a half reaction the amount of a substance that is reduced or oxidized is directly proportional to the number of electrons generated in the cell.
Free
(True/False)
4.9/5  (37)
(37)
Correct Answer:
True
The purpose of the salt bridge in an electrochemical cell is to .
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
B
In a lead- acid battery, the electrodes are consumed. In this battery,
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
E
The balanced half- reaction in which sulfate ion is reduced to sulfite ion is a process.
(Multiple Choice)
4.8/5  (45)
(45)
What is the coefficient of the permanganate ion when the following equation is balanced? 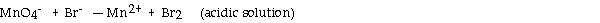
(Multiple Choice)
4.8/5  (31)
(31)
When iron is coated with a thin layer of zinc to protect against corrosion, the iron is said to be .
(Short Answer)
4.9/5  (41)
(41)
Using Table 20.1, which substance can oxidize I- (aq) to I2 (s) ?
(Multiple Choice)
4.8/5  (31)
(31)
What is the oxidation number of manganese in the Mn O 1- ion?
(Multiple Choice)
5.0/5  (35)
(35)
How many seconds are required to produce 1.0 g of silver metal by the electrolysis of a AgNO3 solution using a current of 30 amps _ ?
(Multiple Choice)
4.9/5  (23)
(23)
The standard cell potential (E°cell) for the voltaic cell based on the reaction below is V. 
(Multiple Choice)
4.9/5  (30)
(30)
How many grams of copper will be plated out by a current of 2.3 A applied for 25 minutes to a 0.50- M solution of copper(II) sulfate _ ?
(Multiple Choice)
4.8/5  (34)
(34)
electrons appear in the following half- reaction when it is balanced. 
(Multiple Choice)
4.9/5  (34)
(34)
The more the value of E°red, the greater the driving force for reduction.
(Multiple Choice)
4.7/5  (38)
(38)
A voltaic cell can be constructed of the same species as long as the are different.
(Short Answer)
4.8/5  (31)
(31)
Which transformation could take place at the anode of an electrochemical cell?
(Multiple Choice)
4.9/5  (44)
(44)
The standard cell potential (E°) of a voltaic cell constructed using the cell reaction below is 0.76 V:
![The standard cell potential (E°) of a voltaic cell constructed using the cell reaction below is 0.76 V: With P<sub>H</sub><sub>2 </sub>= 1.0 atm and [Zn<sup>2</sup><sup>+</sup>] = 1.0 M, the cell potential is 0.66 V. The concentration of H<sup>+ </sup>in the Cathode compartment is _ M.](https://storage.examlex.com/TB1819/11ead62c_1f1e_35f4_ac95_0934b47cea8a_TB1819_00.jpg) With PH2 = 1.0 atm and [Zn2+] = 1.0 M, the cell potential is 0.66 V. The concentration of H+ in the
Cathode compartment is _ M.
With PH2 = 1.0 atm and [Zn2+] = 1.0 M, the cell potential is 0.66 V. The concentration of H+ in the
Cathode compartment is _ M.
(Multiple Choice)
4.7/5  (32)
(32)
Showing 1 - 20 of 90
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)