Deck 19: Chemical Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/101
Play
Full screen (f)
Deck 19: Chemical Thermodynamics
1
For a given reaction, OH = +35.5 kJ/mol and OS = +83.6 J/K- mol. The reaction is spontaneous
) Assume that OH and OS do not vary with temperature.
A) at all temperatures
B) at T < 425 K
C) at T < 298 K
D) at T > 425 K
E) at T > 298 K
) Assume that OH and OS do not vary with temperature.
A) at all temperatures
B) at T < 425 K
C) at T < 298 K
D) at T > 425 K
E) at T > 298 K
at T > 425 K
2
OS is positive for the reaction _.
A) 2SO3 (g) - 2SO2 (g) + O2 (g)
B) H2O (l) -H2O (s)
C) CaO (s) + CO2 (g) -CaCO3 (s)
D) Ag+ (aq) + Cl- (aq) - AgCl (s)
E) N2 (g) + 3H2 (g) - 2NH3 (g)
A) 2SO3 (g) - 2SO2 (g) + O2 (g)
B) H2O (l) -H2O (s)
C) CaO (s) + CO2 (g) -CaCO3 (s)
D) Ag+ (aq) + Cl- (aq) - AgCl (s)
E) N2 (g) + 3H2 (g) - 2NH3 (g)
2SO3 (g) - 2SO2 (g) + O2 (g)
3
The equilibrium position corresponds to which letter on the graph of G vs f (course of reaction) below?
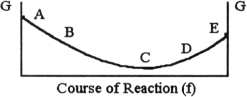
A) A
B) B
C) C
D) D
E) E

A) A
B) B
C) C
D) D
E) E
C
4
In the Haber process, ammonia is synthesized from nitrogen and hydrogen:

ΔG° at 298 oK for this reaction is - 33.3 kJ/mol. The value of ΔG at 298 K for a reaction mixture that consists of 1.9 atm N2, 1.6 atm H2, and 0.65 atm NH3 is _ .
A) - 104.5
B) - 3.86 × 103
C) - 40.5
D) - 7.25 × 103
E) - 1.8

ΔG° at 298 oK for this reaction is - 33.3 kJ/mol. The value of ΔG at 298 K for a reaction mixture that consists of 1.9 atm N2, 1.6 atm H2, and 0.65 atm NH3 is _ .
A) - 104.5
B) - 3.86 × 103
C) - 40.5
D) - 7.25 × 103
E) - 1.8

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
5
A reaction that is spontaneous as written .
A) is very rapid
B) is very slow
C) will proceed without outside intervention
D) is also spontaneous in the reverse direction
E) has an equilibrium position that lies far to the left
A) is very rapid
B) is very slow
C) will proceed without outside intervention
D) is also spontaneous in the reverse direction
E) has an equilibrium position that lies far to the left

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
6
ΔS is negative for the reaction .
A) 2C (s) + O2 (g) - 2CO2 (g)
B) 2SO2 (g) + O2 (g) - 2SO3 (g)
C) H2O (l) -H2O (g)
D) NH4Cl (s) - NH3 (g) + HCl (g)
E) PbCl2 (s) - Pb2+ (aq) + 2Cl- (aq)
A) 2C (s) + O2 (g) - 2CO2 (g)
B) 2SO2 (g) + O2 (g) - 2SO3 (g)
C) H2O (l) -H2O (g)
D) NH4Cl (s) - NH3 (g) + HCl (g)
E) PbCl2 (s) - Pb2+ (aq) + 2Cl- (aq)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
7
The value of ΔG° at 100.0 °C for the formation of calcium chloride from its constituent elements:

Is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 795.8 kJ/mol, ΔG° is - 748.1 kJ/mol, and
ΔS° is - 159.8 J/K.
A) 5.88 × 104
B) - 855.4
C) 1.52 × 104
D) - 779.8
E) - 736.2

Is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 795.8 kJ/mol, ΔG° is - 748.1 kJ/mol, and
ΔS° is - 159.8 J/K.
A) 5.88 × 104
B) - 855.4
C) 1.52 × 104
D) - 779.8
E) - 736.2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
8
With thermodynamics, one cannot determine .
A) the speed of a reaction
B) the extent of a reaction
C) the value of the equilibrium constant
D) the direction of a spontaneous reaction
E) the temperature at which a reaction will be spontaneous
A) the speed of a reaction
B) the extent of a reaction
C) the value of the equilibrium constant
D) the direction of a spontaneous reaction
E) the temperature at which a reaction will be spontaneous

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
9
The entropy of the universe is .
A) constant
B) continually increasing
C) continually decreasing
D) the same as the energy, E
E) zero
A) constant
B) continually increasing
C) continually decreasing
D) the same as the energy, E
E) zero

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
10
The thermodynamic quantity that expresses the degree of disorder in a system is _ _.
A) heat flow
B) enthalpy
C) entropy
D) bond energy
E) internal energy
A) heat flow
B) enthalpy
C) entropy
D) bond energy
E) internal energy

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
11
For the reaction 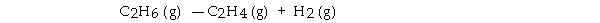 ΔH° is +137 kJ/mol and ΔS° is +120 J/K · mol. This reaction is .
ΔH° is +137 kJ/mol and ΔS° is +120 J/K · mol. This reaction is .
A) spontaneous only at high temperature
B) spontaneous at all temperatures
C) spontaneous only at low temperature
D) nonspontaneous at all temperatures
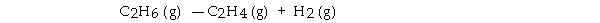 ΔH° is +137 kJ/mol and ΔS° is +120 J/K · mol. This reaction is .
ΔH° is +137 kJ/mol and ΔS° is +120 J/K · mol. This reaction is .A) spontaneous only at high temperature
B) spontaneous at all temperatures
C) spontaneous only at low temperature
D) nonspontaneous at all temperatures

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
12
A common name for methanol (CH3OH) is wood alcohol. The normal boiling point of methanol is 64.7 °C and the molar enthalpy of vaporization if 71.8 kJ/mol. The value of ΔS when 2.15 mol of CH3OH (l) vaporizes at 64.7 °C is J/K.
A) 5.21 × 107
B) 0.457
C) 457
D) 2.39
E) 2.39 × 103
A) 5.21 × 107
B) 0.457
C) 457
D) 2.39
E) 2.39 × 103

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
13
The standard Gibbs free energy of formation of is zero.
(a) H2O (l)
(b) Na (s)
(c) H2 (g)
A) (a) only
B) (b) only
C) (c) only
D) (b) and (c)
E) (a), (b), and (c)
(a) H2O (l)
(b) Na (s)
(c) H2 (g)
A) (a) only
B) (b) only
C) (c) only
D) (b) and (c)
E) (a), (b), and (c)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
14
A system that doesn't exchange matter or energy with its surroundings is called an system.
A) isotonic
B) adiabatic
C) isothermal
D) isobaric
E) isolated
A) isotonic
B) adiabatic
C) isothermal
D) isobaric
E) isolated

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
15
Of the following, only _ is not a state function.
A) H
B) T
C) S
D) q
E) E
A) H
B) T
C) S
D) q
E) E

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the reaction:

Given the following table of thermodynamic data,
Determine the temperature (in °C) above which the reaction is nonspontaneous.
A) This reaction is spontaneous at all temperatures.
B) 618.1
C) 1235
D) 345.1
E) 432.8

Given the following table of thermodynamic data,

Determine the temperature (in °C) above which the reaction is nonspontaneous.
A) This reaction is spontaneous at all temperatures.
B) 618.1
C) 1235
D) 345.1
E) 432.8

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
17
Given the following table of thermodynamic data,

Complete the following sentence. The vaporization of TiCl4 is _ .
A) nonspontaneous at low temperature and spontaneous at high temperature
B) spontaneous at low temperature and nonspontaneous at high temperature
C) spontaneous at all temperatures
D) nonspontaneous at all temperatures
E) not enough information given to draw a conclusion

Complete the following sentence. The vaporization of TiCl4 is _ .
A) nonspontaneous at low temperature and spontaneous at high temperature
B) spontaneous at low temperature and nonspontaneous at high temperature
C) spontaneous at all temperatures
D) nonspontaneous at all temperatures
E) not enough information given to draw a conclusion

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
18
A reversible process is one that _ _.
A) must be carried out at high temperature
B) can be reversed with no net change in either system or surroundings
C) must be carried out at low temperature
D) happens spontaneously
E) is spontaneous in both directions
A) must be carried out at high temperature
B) can be reversed with no net change in either system or surroundings
C) must be carried out at low temperature
D) happens spontaneously
E) is spontaneous in both directions

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
19
Which reaction produces an increase in the entropy of the system?
A) H2 (g) + Cl2 (g) - 2 HCl (g)
B) Ag+ (aq) + Cl- (aq) - AgCl (s)
C) N2 (g) + 3 H2 (g) - 2 NH3 (g)
D) H2O (l) -H2O (s)
E) CO2 (s) - CO2 (g)
A) H2 (g) + Cl2 (g) - 2 HCl (g)
B) Ag+ (aq) + Cl- (aq) - AgCl (s)
C) N2 (g) + 3 H2 (g) - 2 NH3 (g)
D) H2O (l) -H2O (s)
E) CO2 (s) - CO2 (g)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
20
The second law of thermodynamics states that .
A) ΔH°rxn = 3 nΔH°f (products) - 3 mΔH°f (reactants)
B) for any spontaneous process, the entropy of the universe increases
C) ΔS = qrev/T at constant temperature
D) ΔE = q + w
E) the entropy of a pure crystalline substance is zero at absolute zero
A) ΔH°rxn = 3 nΔH°f (products) - 3 mΔH°f (reactants)
B) for any spontaneous process, the entropy of the universe increases
C) ΔS = qrev/T at constant temperature
D) ΔE = q + w
E) the entropy of a pure crystalline substance is zero at absolute zero

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
21
A reaction that is not spontaneous at low temperature can become spontaneous at high temperature if OH is and OS is _ .
A) +, +
B) - , -
C) +, -
D) - , +
E) +, 0
A) +, +
B) - , -
C) +, -
D) - , +
E) +, 0

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
22
Which one of the following is always positive when a spontaneous process occurs?
A) ΔH universe
B) ΔS universe
C) ΔS surroundings
D) ΔH surroundings
E) ΔS system
A) ΔH universe
B) ΔS universe
C) ΔS surroundings
D) ΔH surroundings
E) ΔS system

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
23
The standard Gibbs free energy of formation of is zero.
(a) H2O (l)
(b) O (g)
(c) H2 (g)
A) (a) only
B) (b) only
C) (c) only
D) (b) and (c)
E) (a), (b), and (c)
(a) H2O (l)
(b) O (g)
(c) H2 (g)
A) (a) only
B) (b) only
C) (c) only
D) (b) and (c)
E) (a), (b), and (c)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
24
Which one of the following statements is true about the equilibrium constant for a reaction if ΔG° for the reaction is negative?
A) K = 1
B) K > 1
C) K < 1
D) K = 0
E) More information is needed.
A) K = 1
B) K > 1
C) K < 1
D) K = 0
E) More information is needed.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
25
Given the following table of thermodynamic data,

Complete the following sentence. The vaporization of PCl3 (l) is .
A) spontaneous at low temperature and nonspontaneous at high temperature
B) nonspontaneous at all temperatures
C) spontaneous at all temperatures
D) nonspontaneous at low temperature and spontaneous at high temperature
E) not enough information given to draw a conclusion

Complete the following sentence. The vaporization of PCl3 (l) is .
A) spontaneous at low temperature and nonspontaneous at high temperature
B) nonspontaneous at all temperatures
C) spontaneous at all temperatures
D) nonspontaneous at low temperature and spontaneous at high temperature
E) not enough information given to draw a conclusion

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
26
For an isothermal process, OS = .
A) Tqrev
B) q
C) qrev/T
D) q + w
E) qrev
A) Tqrev
B) q
C) qrev/T
D) q + w
E) qrev

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
27
When a system is at equilibrium, _.
A) the process is not spontaneous in either direction
B) the forward and the reverse processes are both spontaneous
C) both forward and reverse processes have stopped
D) the reverse process is spontaneous but the forward process is not
E) the forward process is spontaneous but the reverse process is not
A) the process is not spontaneous in either direction
B) the forward and the reverse processes are both spontaneous
C) both forward and reverse processes have stopped
D) the reverse process is spontaneous but the forward process is not
E) the forward process is spontaneous but the reverse process is not

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
28
For a reaction to be spontaneous under standard conditions at all temperatures, the signs of ΔH° and ΔS° must be _ _ and , respectively.
A) +, +
B) +, -
C) - , +
D) - , -
E) +, 0
A) +, +
B) +, -
C) - , +
D) - , -
E) +, 0

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
29
Which one of the following correctly indicates the relationship between the entropy of a system and the number of different arrangements, W, in the system?
A) S = k lnW
B)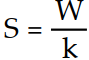
C)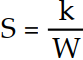
D) S = kW
E) S = Wk
A) S = k lnW
B)
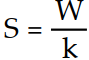
C)
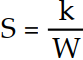
D) S = kW
E) S = Wk

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
30
The value of ΔG° at 141.0 °C for the formation of phosphorous trichloride from its constituent elements, 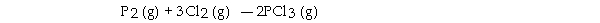 is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 720.5 kJ/mol, ΔG° is - 642.9 kJ/mol, and
is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 720.5 kJ/mol, ΔG° is - 642.9 kJ/mol, and
ΔS° is - 263.7 J/K.
A) - 611.3
B) - 829.7
C) 3.65 × 104
D) 1.08 × 105
E) - 683.3
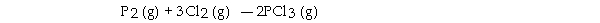 is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 720.5 kJ/mol, ΔG° is - 642.9 kJ/mol, and
is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 720.5 kJ/mol, ΔG° is - 642.9 kJ/mol, andΔS° is - 263.7 J/K.
A) - 611.3
B) - 829.7
C) 3.65 × 104
D) 1.08 × 105
E) - 683.3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
31
The first law of thermodynamics can be given as .
A) for any spontaneous process, the entropy of the universe increases
B) ΔS = qrev/T at constant temperature
C) ΔE = q + w
D) the entropy of a pure crystalline substance at absolute zero is zero
E) ΔH°rxn = ΣnΔH°f (products) - ΣmΔH°f (reactants)
A) for any spontaneous process, the entropy of the universe increases
B) ΔS = qrev/T at constant temperature
C) ΔE = q + w
D) the entropy of a pure crystalline substance at absolute zero is zero
E) ΔH°rxn = ΣnΔH°f (products) - ΣmΔH°f (reactants)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
32
OS is positive for the reaction _.
A) 2Hg (l) + O2 (g) - 2HgO (s)
B) BaF2 (s) - Ba2+ (aq) + 2F- (aq)
C) 2H2 (g) + O2 (g) - 2H2O (g)
D) 2NO2 (g) - N2O4 (g)
E) CO2 (g) -CO2 (s)
A) 2Hg (l) + O2 (g) - 2HgO (s)
B) BaF2 (s) - Ba2+ (aq) + 2F- (aq)
C) 2H2 (g) + O2 (g) - 2H2O (g)
D) 2NO2 (g) - N2O4 (g)
E) CO2 (g) -CO2 (s)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
33
The value of ΔG° at 100.0 °C for the oxidation of solid elemental sulfur to gaseous sulfur dioxide,
S (s,rhombic) + O2 (g) →SO2 (g)
Is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 269.9 kJ/mol, ΔG° is - 300.4 kJ/mol, and
ΔS° is +11.6 J/K.
A) - 274.2
B) - 1,430
C) - 4,598
D) - 271.1
E) - 265.6
S (s,rhombic) + O2 (g) →SO2 (g)
Is kJ/mol. At 25.0 oC for this reaction, ΔH° is - 269.9 kJ/mol, ΔG° is - 300.4 kJ/mol, and
ΔS° is +11.6 J/K.
A) - 274.2
B) - 1,430
C) - 4,598
D) - 271.1
E) - 265.6

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
34
Of the following, the entropy of gaseous _ is the largest at 25 °C and 1 atm.
A) C2H4
B) C2H2
C) C2H6
D) H2
E) CH4
A) C2H4
B) C2H2
C) C2H6
D) H2
E) CH4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
35
Which one of the following processes produces a decrease in the entropy of the system?
A) boiling water to form steam
B) melting ice to form water
C) dissolution of solid KCl in water
D) mixing of two gases into one container
E) freezing water to form ice
A) boiling water to form steam
B) melting ice to form water
C) dissolution of solid KCl in water
D) mixing of two gases into one container
E) freezing water to form ice

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
36
For a given reaction, ΔH = - 19.9 kJ/mol and ΔS = - 55.5 J/K- mol. The reaction will have ΔG = 0 at------K. Assume that ΔH and ΔS do not vary with temperature.
A) 359
B) 2789
C) 0.359
D) 2.79
E) 298
A) 359
B) 2789
C) 0.359
D) 2.79
E) 298

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
37
If ΔG° for a reaction is greater than zero, then .
A) K = 1
B) K = 0
C) K < 1
D) K > 1
E) More information is needed.
A) K = 1
B) K = 0
C) K < 1
D) K > 1
E) More information is needed.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
38
Of the following, the entropy of is the largest.
A) HCl (g)
B) HCl (s)
C) HCl (l)
D) HBr (g)
E) HI (g)
A) HCl (g)
B) HCl (s)
C) HCl (l)
D) HBr (g)
E) HI (g)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
39
Which reaction produces a decrease in the entropy of the system?
A) 2C (s) + O2 (g) - 2CO (g)
B) CO2 (s) -CO2 (g)
C) H2O (l) -H2O (g)
D) 2H2 (g) + O2 (g) - 2H2O (l)
E) CaCO3 (s) - CaO (s) + CO2 (g)
A) 2C (s) + O2 (g) - 2CO (g)
B) CO2 (s) -CO2 (g)
C) H2O (l) -H2O (g)
D) 2H2 (g) + O2 (g) - 2H2O (l)
E) CaCO3 (s) - CaO (s) + CO2 (g)

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
40
Phosphorous and chlorine gases combine to produce phosphorous trichloride:

ΔG° at 298 oK for this reaction is - 642.9 kJ/mol. The value of ΔG at 298 K for a reaction mixture that consists of 1.5 atm P2, 1.6 atm Cl2, and 0.65 atm PCl3 is _ .
A) - 7.28 × 103
B) - 3.88 × 103
C) - 649.5
D) - 44.2
E) - 708.4

ΔG° at 298 oK for this reaction is - 642.9 kJ/mol. The value of ΔG at 298 K for a reaction mixture that consists of 1.5 atm P2, 1.6 atm Cl2, and 0.65 atm PCl3 is _ .
A) - 7.28 × 103
B) - 3.88 × 103
C) - 649.5
D) - 44.2
E) - 708.4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
41
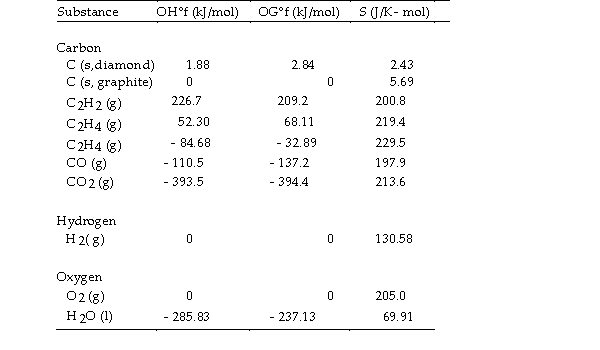
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)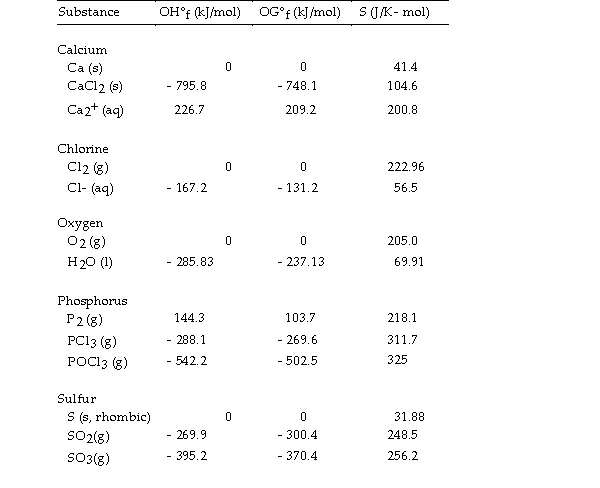
The value of ΔG°at 25 °C for the decomposition of phosphorous trichloride into its constituent elements,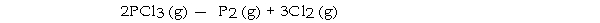 is kJ/mol.
is kJ/mol.
A) +642.9
B) - 373.3
C) - 539.2
D) - 642.9
E) +539.2
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔG°at 25 °C for the decomposition of phosphorous trichloride into its constituent elements,
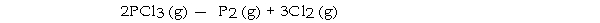 is kJ/mol.
is kJ/mol.A) +642.9
B) - 373.3
C) - 539.2
D) - 642.9
E) +539.2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
42
Find the temperature above which a reaction with a OH of 53 .00 kJ/mol and a OS of 100.0 J/K·mol becomes spontaneous.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
43
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
 The combustion of acetylene in the presence of excess oxygen yields carbon dioxide
The combustion of acetylene in the presence of excess oxygen yields carbon dioxide 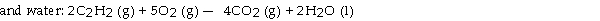
The value of ΔSo for this reaction is J/K.
A) +432.4
B) +689.3
C) - 122.3
D) +122.3
E) - 432.4
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) The combustion of acetylene in the presence of excess oxygen yields carbon dioxide
The combustion of acetylene in the presence of excess oxygen yields carbon dioxide 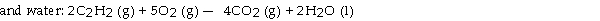
The value of ΔSo for this reaction is J/K.
A) +432.4
B) +689.3
C) - 122.3
D) +122.3
E) - 432.4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
44
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔH° for the decomposition of phosphorous trichloride into its constituent elements, is kJ/mol.
is kJ/mol.
A) - 288.1
B) - 720.5
C) +720.5
D) +288.1
E) +576.2
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔH° for the decomposition of phosphorous trichloride into its constituent elements,
 is kJ/mol.
is kJ/mol.A) - 288.1
B) - 720.5
C) +720.5
D) +288.1
E) +576.2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate OG○(in kJ/mol) for the following reaction at 1 atm and 25○C: 
OHf○C2H6 (g) = - 84.7 kJ/mol; S○C2H6 (g) = 229.5 J/K · mol; OHf○CO2 (g) = - 393.5
kJ/mol; S○CO2 (g) = 213.6 J/K · mol; OHf○H2O (l) = - 285.8 kJ/mol; S○H2O (l) = 69.9 J/K ·
mol

OHf○C2H6 (g) = - 84.7 kJ/mol; S○C2H6 (g) = 229.5 J/K · mol; OHf○CO2 (g) = - 393.5
kJ/mol; S○CO2 (g) = 213.6 J/K · mol; OHf○H2O (l) = - 285.8 kJ/mol; S○H2O (l) = 69.9 J/K ·
mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
46
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide, is kJ/mol.
is kJ/mol.
A) - 395.2
B) +395.2
C) - 790.4
D) +105.1
E) +790.4
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,
 is kJ/mol.
is kJ/mol.A) - 395.2
B) +395.2
C) - 790.4
D) +105.1
E) +790.4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
47
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔG° at 25 °C for the formation of POCl3 from its constituent elements, is kJ/mol.
is kJ/mol.
A) - 606.2
B) - 1,005
C) +606.2
D) - 1,109
E) +1,109
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔG° at 25 °C for the formation of POCl3 from its constituent elements,
 is kJ/mol.
is kJ/mol.A) - 606.2
B) - 1,005
C) +606.2
D) - 1,109
E) +1,109

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
48
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔH° for the decomposition of POCl3 into its constituent elements, is kJ/mol.
is kJ/mol.
A) - 940.1
B) +940.1
C) - 1,228.7
D) +1,228.7
E) +0.00
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔH° for the decomposition of POCl3 into its constituent elements,
 is kJ/mol.
is kJ/mol.A) - 940.1
B) +940.1
C) - 1,228.7
D) +1,228.7
E) +0.00

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
49
Calculate OGo (in kJ/mol) for the following reaction at 1 atm and 25○C: 
OGf○C2H6 (g) = - 32.89 kJ/mol; OGf○CO2 (g) = - 394.4 kJ/mol; OGf○H2O (l) = - 237.2
kJ/mol

OGf○C2H6 (g) = - 32.89 kJ/mol; OGf○CO2 (g) = - 394.4 kJ/mol; OGf○H2O (l) = - 237.2
kJ/mol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
50
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔS° for the formation of phosphorous trichloride from its constituent elements, is J/K.
is J/K.
A) +129.4
B) +311.7
C) - 263.7
D) - 129.4
E) - 311.7
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔS° for the formation of phosphorous trichloride from its constituent elements,
 is J/K.
is J/K.A) +129.4
B) +311.7
C) - 263.7
D) - 129.4
E) - 311.7

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
51
For the reaction 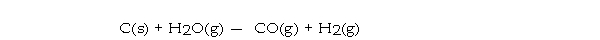 ΔH° = 131.3 kJ/mol andΔS° = 133.6 J/K · mol at 298 K. At temperatures greater than _°C this reaction is spontaneous under standard conditions.
ΔH° = 131.3 kJ/mol andΔS° = 133.6 J/K · mol at 298 K. At temperatures greater than _°C this reaction is spontaneous under standard conditions.
A) 710
B) 983
C) 325
D) 273
E) 552
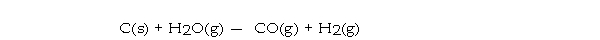 ΔH° = 131.3 kJ/mol andΔS° = 133.6 J/K · mol at 298 K. At temperatures greater than _°C this reaction is spontaneous under standard conditions.
ΔH° = 131.3 kJ/mol andΔS° = 133.6 J/K · mol at 298 K. At temperatures greater than _°C this reaction is spontaneous under standard conditions.A) 710
B) 983
C) 325
D) 273
E) 552

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
52
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔS° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide, is J/K.
is J/K.
A) - 166.4
B) +493.1
C) - 19.3
D) - 493.1
E) +19.3
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔS° for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,
 is J/K.
is J/K.A) - 166.4
B) +493.1
C) - 19.3
D) - 493.1
E) +19.3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
53
Consider a pure crystalline solid that is heated from absolute zero to a temperature above the boiling point of the liquid. Which of the following processes produces the greatest increase in the entropy of the substance?
A) melting the solid
B) heating the solid
C) vaporizing the liquid
D) heating the gas
E) heating the liquid
A) melting the solid
B) heating the solid
C) vaporizing the liquid
D) heating the gas
E) heating the liquid

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the reaction:
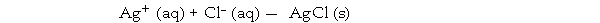
Given the following table of thermodynamic data,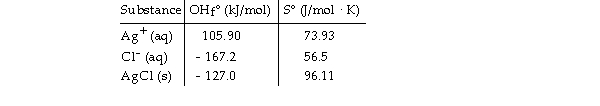
Determine the temperature (in °C) above which the reaction is nonspontaneous under standard conditions.
A) 1641
B) 432.8
C) 1235
D) 150.5
E) 133.0
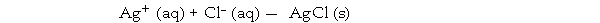
Given the following table of thermodynamic data,

Determine the temperature (in °C) above which the reaction is nonspontaneous under standard conditions.
A) 1641
B) 432.8
C) 1235
D) 150.5
E) 133.0

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
55
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, is kJ/mol.
is kJ/mol.
A) - 269.9
B) +11.6
C) - 11.6
D) +269.9
E) +0.00
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔH° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide,
 is kJ/mol.
is kJ/mol.A) - 269.9
B) +11.6
C) - 11.6
D) +269.9
E) +0.00

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
56
Calculate OG○for the autoionization of water at 25○C. Kw = 1.0 × 10- 14

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
57
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔH° for the formation of POCl3 from its constituent elements, is kJ/mol.
is kJ/mol.
A) - 686.5
B) +1228.7
C) +686.5
D) - 1228.7
E) - 397.7
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔH° for the formation of POCl3 from its constituent elements,
 is kJ/mol.
is kJ/mol.A) - 686.5
B) +1228.7
C) +686.5
D) - 1228.7
E) - 397.7

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
58
Which one of the following processes produces a decrease of the entropy of the system?
A) dissolving sodium chloride in water
B) sublimation of naphthalene
C) dissolving oxygen in water
D) explosion of nitroglycerine
E) boiling of alcohol
A) dissolving sodium chloride in water
B) sublimation of naphthalene
C) dissolving oxygen in water
D) explosion of nitroglycerine
E) boiling of alcohol

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the reaction:
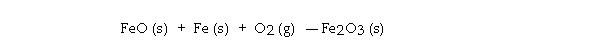
Given the following table of thermodynamic data,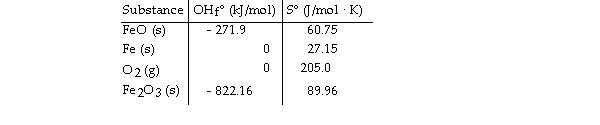
Determine the temperature (in °C) above which the reaction is nonspontaneous.
A) 756.3
B) 2439
C) This reaction is spontaneous at all temperatures.
D) 618.1
E) 1235
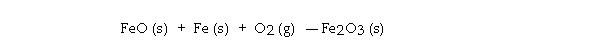
Given the following table of thermodynamic data,

Determine the temperature (in °C) above which the reaction is nonspontaneous.
A) 756.3
B) 2439
C) This reaction is spontaneous at all temperatures.
D) 618.1
E) 1235

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
60
Find the temperature above which a reaction with a OH of 123 .0 kJ/mol and a OS of 90.00 J/K·mol becomes spontaneous.

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
61
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)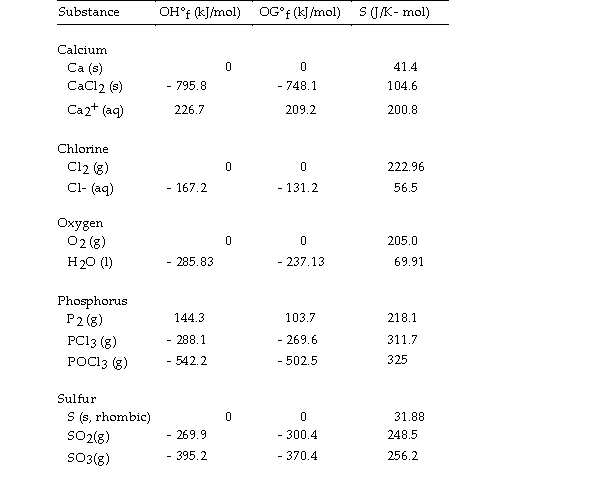
The value of ΔG° at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur trioxide, is kJ/mol.
is kJ/mol.
A) +370.4
B) - 740.8
C) +740.8
D) +185.2
E) - 370.4
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔG° at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur trioxide,
 is kJ/mol.
is kJ/mol.A) +370.4
B) - 740.8
C) +740.8
D) +185.2
E) - 370.4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
62
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 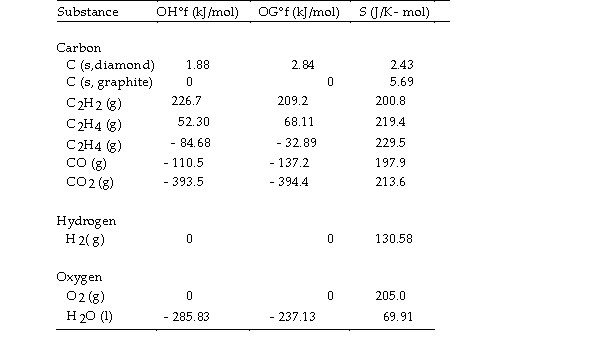
The value of ΔS° for the catalytic hydrogenation of acetylene to ethane, is J/K.
is J/K.
A) +28.7
B) +232.5
C) - 232.5
D) +440.9
E) - 76.0
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 
The value of ΔS° for the catalytic hydrogenation of acetylene to ethane,
 is J/K.
is J/K.A) +28.7
B) +232.5
C) - 232.5
D) +440.9
E) - 76.0

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
63
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)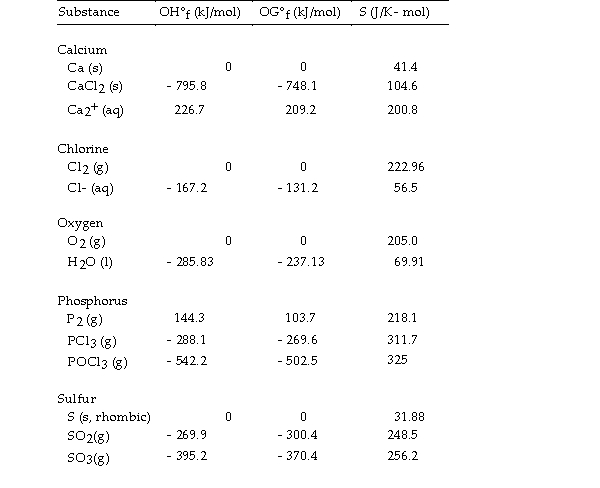
The value of ΔS° for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen, is J/K.
is J/K.
A) - 19.3
B) +493.1
C) +19.3
D) +166.4
E) - 493.1
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔS° for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen,
 is J/K.
is J/K.A) - 19.3
B) +493.1
C) +19.3
D) +166.4
E) - 493.1

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
64
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 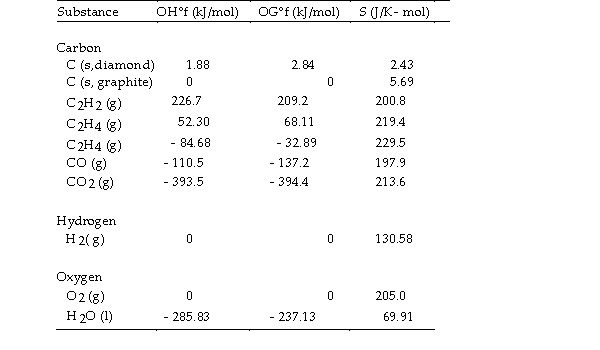
The combustion of ethene in the presence of excess oxygen yields carbon dioxide and water:

The value of OSo for this reaction is J/K.
A) - 347.6
B) +140.9
C) - 140.9
D) +347.6
E) - 267.4
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 
The combustion of ethene in the presence of excess oxygen yields carbon dioxide and water:

The value of OSo for this reaction is J/K.
A) - 347.6
B) +140.9
C) - 140.9
D) +347.6
E) - 267.4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
65
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)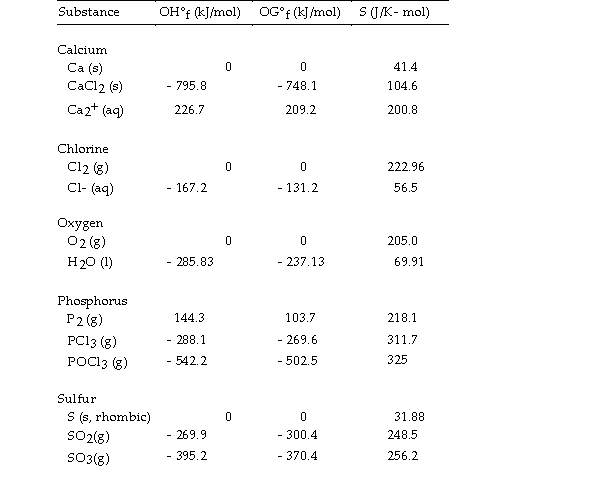
The combustion of hydrogen in the presence of excess oxygen yields water:

The value of OSo for this reaction is J/K.
A) +265.7
B) +405.5
C) - 405.5
D) - 265.7
E) - 326.3
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The combustion of hydrogen in the presence of excess oxygen yields water:

The value of OSo for this reaction is J/K.
A) +265.7
B) +405.5
C) - 405.5
D) - 265.7
E) - 326.3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
66
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)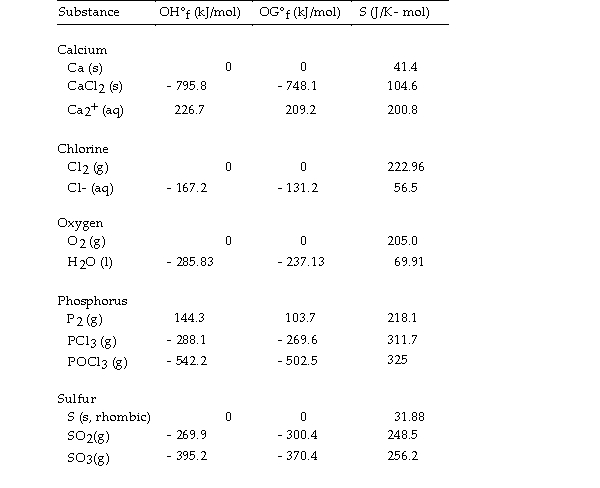
The value of ΔS° for the decomposition of calcium chloride into its constituent elements, is J/K.
is J/K.
A) +159.8
B) +104.6
C) - 159.8
D) - 104.6
E) +369.0
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔS° for the decomposition of calcium chloride into its constituent elements,
 is J/K.
is J/K.A) +159.8
B) +104.6
C) - 159.8
D) - 104.6
E) +369.0

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
67
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)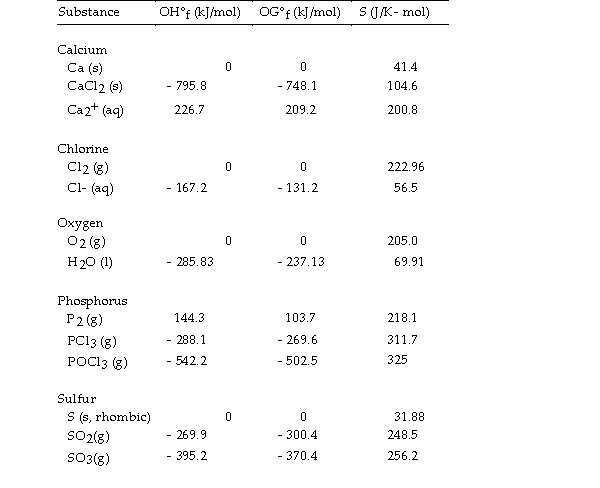
The value of ΔH° for the decomposition of calcium chloride into its constituent elements, is kJ/mol.
is kJ/mol.
A) - 795.8
B) +397.9
C) - 0.00
D) +795.8
E) - 397.9
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔH° for the decomposition of calcium chloride into its constituent elements,
 is kJ/mol.
is kJ/mol.A) - 795.8
B) +397.9
C) - 0.00
D) +795.8
E) - 397.9

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
68
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔG° at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur dioxide, is kJ/mol.
is kJ/mol.
A) - 300.4
B) +300.4
C) +269.9
D) - 269.9
E) +395.2
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔG° at 25 °C for the oxidation of solid elemental sulfur to gaseous sulfur dioxide,
 is kJ/mol.
is kJ/mol.A) - 300.4
B) +300.4
C) +269.9
D) - 269.9
E) +395.2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
69
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔS° for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, is J/K.
is J/K.
A) +11.6
B) - 11.6
C) - 248.5
D) +485.4
E) +248.5
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔS° for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen,
 is J/K.
is J/K.A) +11.6
B) - 11.6
C) - 248.5
D) +485.4
E) +248.5

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
70
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 
Consider the reaction:
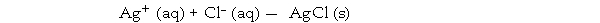
Given the following table of thermodynamic data at 298 oK:
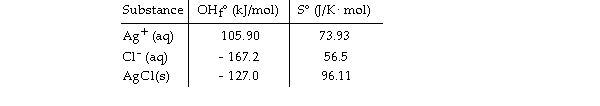
The value of K for the reaction at 25 °C is .
A) 1.8 × 104
B) 1.9 × 10- 10
C) 5.4 × 109
D) 810
E) 3.7 × 1010
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 
Consider the reaction:
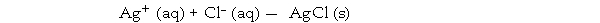
Given the following table of thermodynamic data at 298 oK:

The value of K for the reaction at 25 °C is .
A) 1.8 × 104
B) 1.9 × 10- 10
C) 5.4 × 109
D) 810
E) 3.7 × 1010

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
71
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)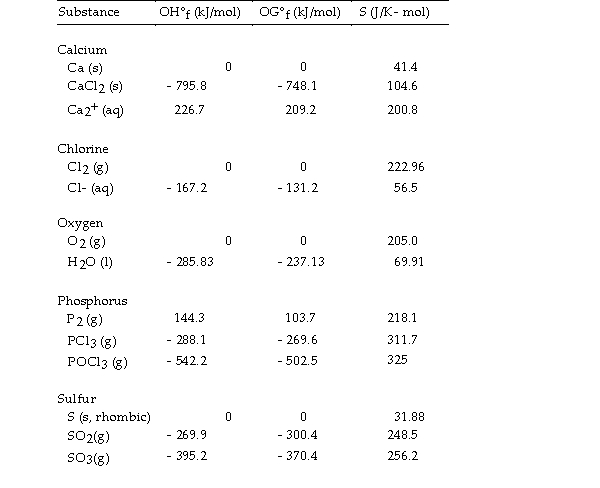
The value of ΔH° for the decomposition of gaseous sulfur trioxide to its component elements, is kJ/mol.
is kJ/mol.
A) +790.4
B) +395.2
C) +105.1
D) - 395.2
E) - 790.4
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔH° for the decomposition of gaseous sulfur trioxide to its component elements,
 is kJ/mol.
is kJ/mol.A) +790.4
B) +395.2
C) +105.1
D) - 395.2
E) - 790.4

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
72
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 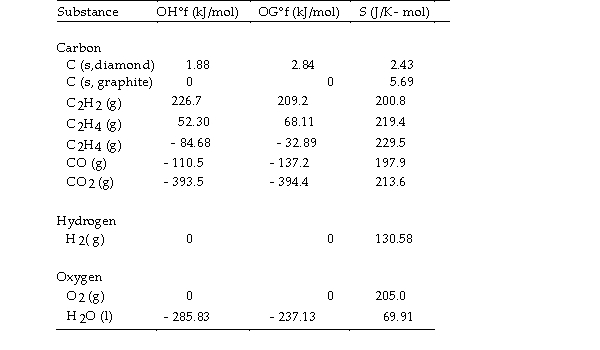
The value of ΔS° for the oxidation of carbon to carbon monoxide, 2C (s, graphite) + O2 (g) -2CO (g)
Is J/K. Carbon monoxide is produced in the combustion of carbon with limited oxygen.
A) +408.6
B) +395.8
C) - 408.6
D) +179.4
E) - 12.8
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 
The value of ΔS° for the oxidation of carbon to carbon monoxide, 2C (s, graphite) + O2 (g) -2CO (g)
Is J/K. Carbon monoxide is produced in the combustion of carbon with limited oxygen.
A) +408.6
B) +395.8
C) - 408.6
D) +179.4
E) - 12.8

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
73
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
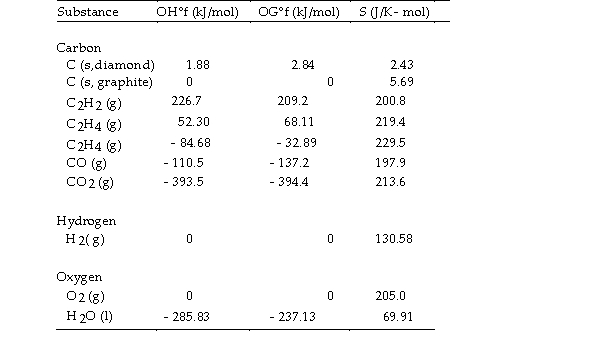 The value of ΔS° for the catalytic hydrogenation of acetylene
The value of ΔS° for the catalytic hydrogenation of acetylene 
Is J/K.
A) +550.8
B) +18.6
C) - 18.6
D) +112.0
E) - 112.0
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) The value of ΔS° for the catalytic hydrogenation of acetylene
The value of ΔS° for the catalytic hydrogenation of acetylene 
Is J/K.
A) +550.8
B) +18.6
C) - 18.6
D) +112.0
E) - 112.0

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
74
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)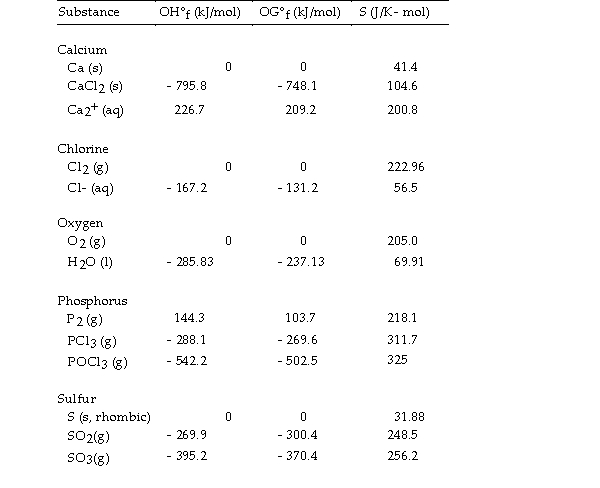
The value of OH° for the formation of phosphorous trichloride from its constituent elements, is kJ/mol
is kJ/mol
A) - 720.5
B) +432.4
C) - 288.1
D) - 432.4
E) +720.5
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of OH° for the formation of phosphorous trichloride from its constituent elements,
 is kJ/mol
is kJ/molA) - 720.5
B) +432.4
C) - 288.1
D) - 432.4
E) +720.5

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
75
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔG° at 25 oC for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen, is kJ/mol.
is kJ/mol.
A) +740.8
B) - 740.8
C) - 370.4
D) +370.4
E) +185.2
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔG° at 25 oC for the decomposition of gaseous sulfur trioxide to solid elemental sulfur and gaseous oxygen,
 is kJ/mol.
is kJ/mol.A) +740.8
B) - 740.8
C) - 370.4
D) +370.4
E) +185.2

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
76
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of OS° for the formation of POCl3 from its constituent elements, is J/K.
is J/K.
A) - 321
B) - 771
C) +321
D) +771
E) - 442
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of OS° for the formation of POCl3 from its constituent elements,
 is J/K.
is J/K.A) - 321
B) - 771
C) +321
D) +771
E) - 442

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
77
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔS° for the reaction
2C (s, diamond) + O2 (g) - 2CO (g) is J/K.
A) +9.5
B) - 195.7
C) - 9.5
D) - 185.9
E) +185.9
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔS° for the reaction
2C (s, diamond) + O2 (g) - 2CO (g) is J/K.
A) +9.5
B) - 195.7
C) - 9.5
D) - 185.9
E) +185.9

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
78
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔS° for the catalytic hydrogenation of ethene to ethane, is J/K.
is J/K.
A) +101.9
B) +112.0
C) - 101.9
D) - 120.5
E) - 232.5
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)The value of ΔS° for the catalytic hydrogenation of ethene to ethane,
 is J/K.
is J/K.A) +101.9
B) +112.0
C) - 101.9
D) - 120.5
E) - 232.5

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
79
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
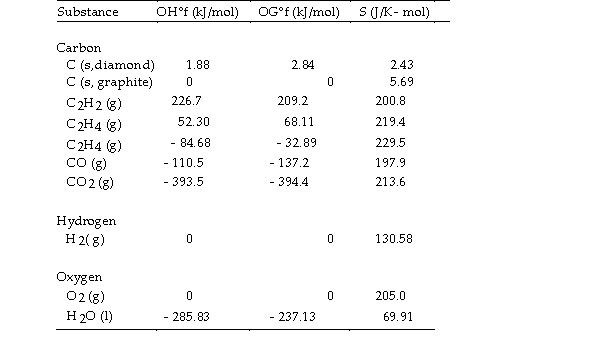 The value of ΔS° for the oxidation of carbon to carbon
The value of ΔS° for the oxidation of carbon to carbon 
Is J/K. The combustion of carbon, as in charcoal briquettes, in the presence of abundant oxygen produces carbon dioxide.
A) +2.9
B) - 2.9
C) - 205.0
D) +205.0
E) +424.3
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) The value of ΔS° for the oxidation of carbon to carbon
The value of ΔS° for the oxidation of carbon to carbon 
Is J/K. The combustion of carbon, as in charcoal briquettes, in the presence of abundant oxygen produces carbon dioxide.
A) +2.9
B) - 2.9
C) - 205.0
D) +205.0
E) +424.3

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck
80
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
The value of ΔG° at 25 °C for the decomposition of POCl3 into its constituent elements, is kJ/mol.
is kJ/mol.
A) +606.2
B) - 1,109
C) +1,109
D) - 606.2
E) - 1,005
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)

The value of ΔG° at 25 °C for the decomposition of POCl3 into its constituent elements,
 is kJ/mol.
is kJ/mol.A) +606.2
B) - 1,109
C) +1,109
D) - 606.2
E) - 1,005

Unlock Deck
Unlock for access to all 101 flashcards in this deck.
Unlock Deck
k this deck


