Exam 19: Chemical Thermodynamics
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 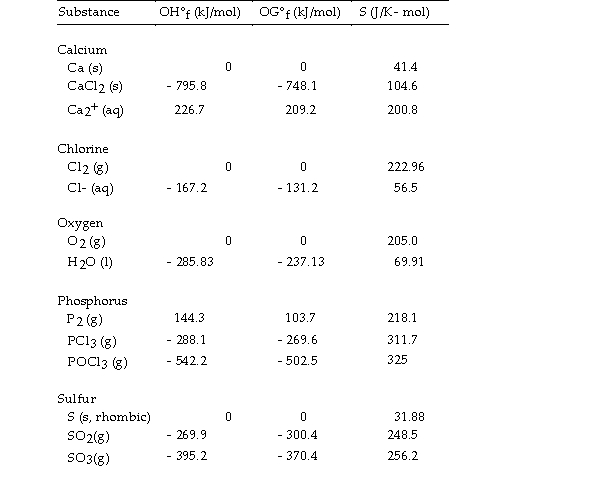 -The value of ΔG° at 25 °C for the decomposition of calcium chloride into its constituent elements,
-The value of ΔG° at 25 °C for the decomposition of calcium chloride into its constituent elements,  is kJ/mol.
is kJ/mol.
Free
(Multiple Choice)
5.0/5  (30)
(30)
Correct Answer:
B
For a reaction to be spontaneous under standard conditions at all temperatures, the signs of ΔH° and ΔS° must be _ _ and , respectively.
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
C
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 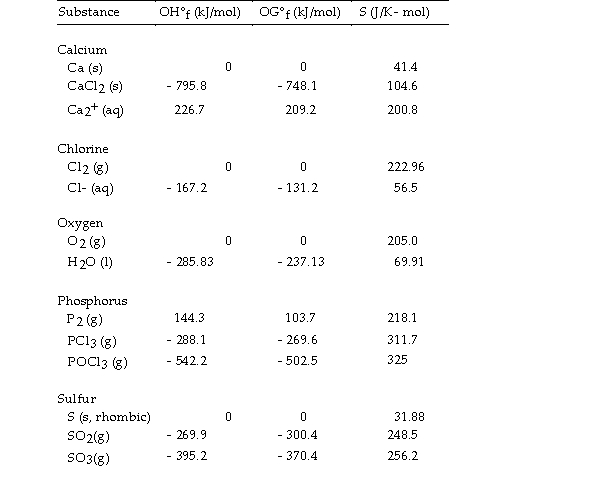 -The value of ΔG° at 25 °C for the formation of calcium chloride from its constituent elements,
-The value of ΔG° at 25 °C for the formation of calcium chloride from its constituent elements,  is kJ/mol.
is kJ/mol.
Free
(Multiple Choice)
4.8/5  (36)
(36)
Correct Answer:
E
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 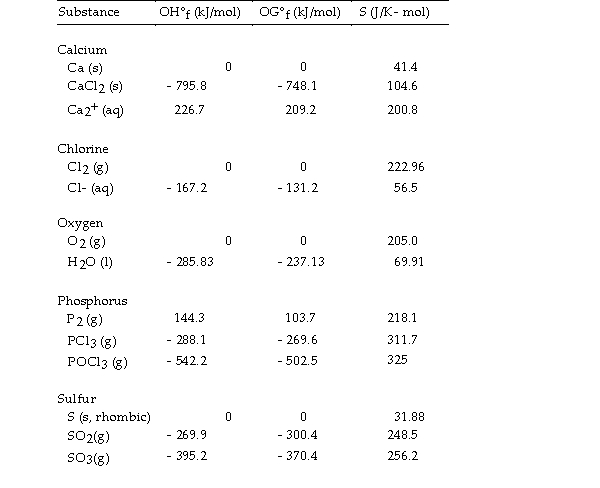 -The value of ΔH° for the decomposition of gaseous sulfur trioxide to its component elements,
-The value of ΔH° for the decomposition of gaseous sulfur trioxide to its component elements,  is kJ/mol.
is kJ/mol.
(Multiple Choice)
4.9/5  (29)
(29)
Of the following, the entropy of gaseous _ is the largest at 25 °C and 1 atm.
(Multiple Choice)
4.8/5  (30)
(30)
For the reaction 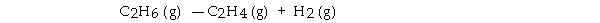 ΔH° is +137 kJ/mol and ΔS° is +120 J/K · mol. This reaction is .
ΔH° is +137 kJ/mol and ΔS° is +120 J/K · mol. This reaction is .
(Multiple Choice)
4.8/5  (35)
(35)
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 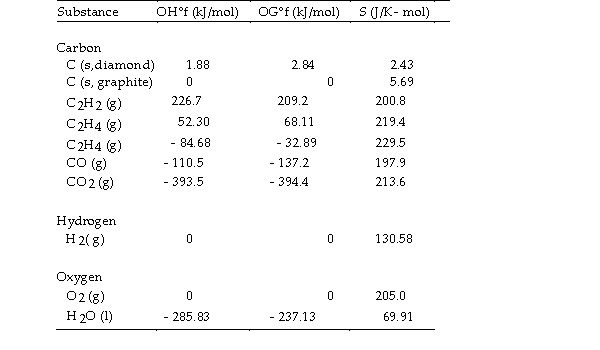 -The combustion of ethene in the presence of excess oxygen yields carbon dioxide and water:
-The combustion of ethene in the presence of excess oxygen yields carbon dioxide and water:
 The value of OSo for this reaction is J/K.
The value of OSo for this reaction is J/K.
(Multiple Choice)
4.9/5  (32)
(32)
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 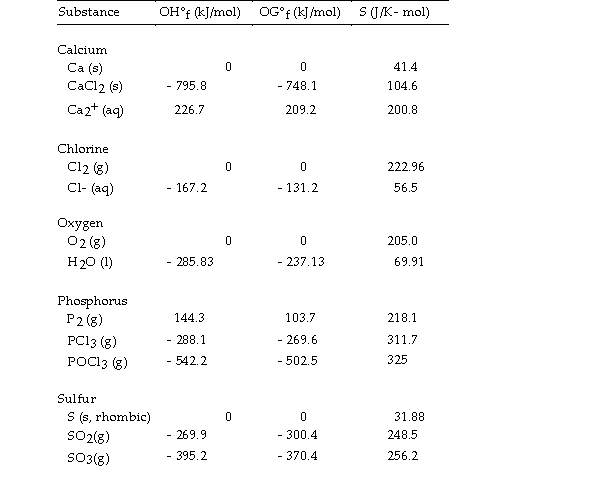 -The value of ΔS° for the reaction
2C (s, diamond) + O2 (g) - 2CO (g) is J/K.
-The value of ΔS° for the reaction
2C (s, diamond) + O2 (g) - 2CO (g) is J/K.
(Multiple Choice)
4.8/5  (35)
(35)
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)  -The value of ΔS° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide,
-The value of ΔS° for the oxidation of solid elemental sulfur to gaseous sulfur dioxide,  is J/K.
is J/K.
(Multiple Choice)
4.9/5  (36)
(36)
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
-
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
-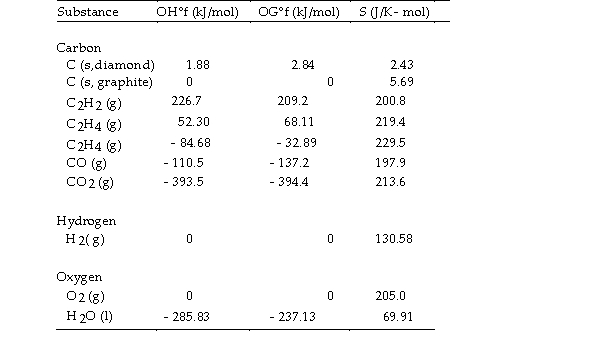 The value of ΔS° for the catalytic hydrogenation of acetylene
The value of ΔS° for the catalytic hydrogenation of acetylene  Is J/K.
Is J/K.
(Multiple Choice)
4.9/5  (31)
(31)
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 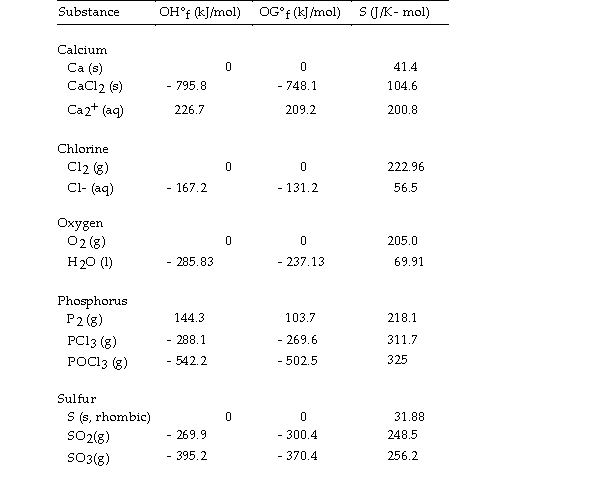 -The value of ΔH° for the formation of calcium chloride from its constituent elements,
-The value of ΔH° for the formation of calcium chloride from its constituent elements,  is kJ/mol.
is kJ/mol.
(Multiple Choice)
4.8/5  (36)
(36)
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 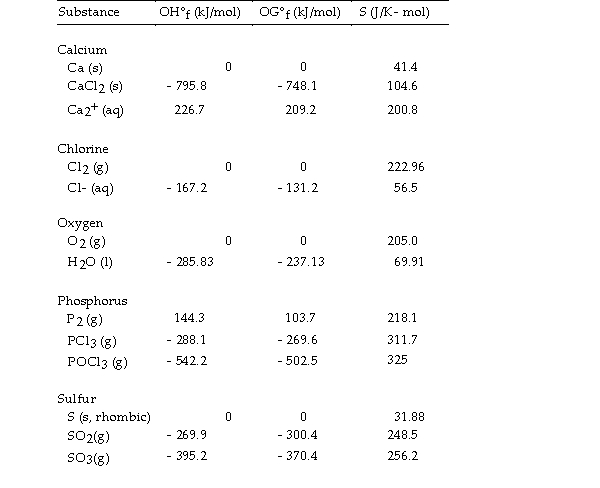 -The value of OS° for the formation of POCl3 from its constituent elements,
-The value of OS° for the formation of POCl3 from its constituent elements,  is J/K.
is J/K.
(Multiple Choice)
4.8/5  (34)
(34)
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C) 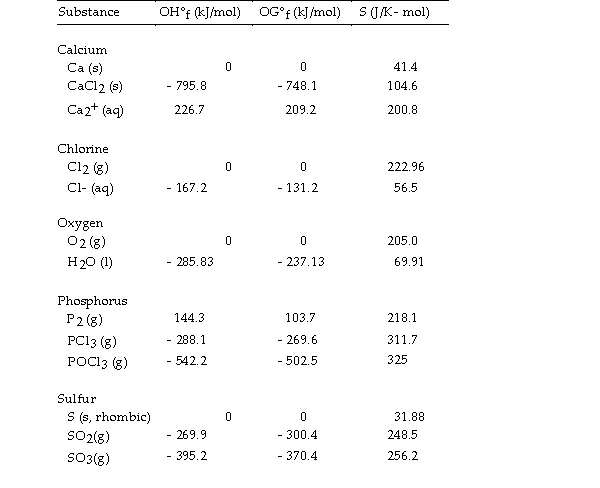 -The value of ΔS° for the formation of phosphorous trichloride from its constituent elements,
-The value of ΔS° for the formation of phosphorous trichloride from its constituent elements,  is J/K.
is J/K.
(Multiple Choice)
4.9/5  (38)
(38)
Use the table below to answer the questions that follow.
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)  -The value of ΔS° for the formation of calcium chloride from its constituent elements,
-The value of ΔS° for the formation of calcium chloride from its constituent elements,  is J/K.
is J/K.
(Multiple Choice)
4.9/5  (32)
(32)
A common name for methanol (CH3OH) is wood alcohol. The normal boiling point of methanol is 64.7 °C and the molar enthalpy of vaporization if 71.8 kJ/mol. The value of ΔS when 2.15 mol of CH3OH (l) vaporizes at 64.7 °C is J/K.
(Multiple Choice)
4.8/5  (37)
(37)
Use the table below to answer the questions that follow.
 Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
-
Thermodynamic Quantities for Selected Substances at 298.15 K (25°C)
-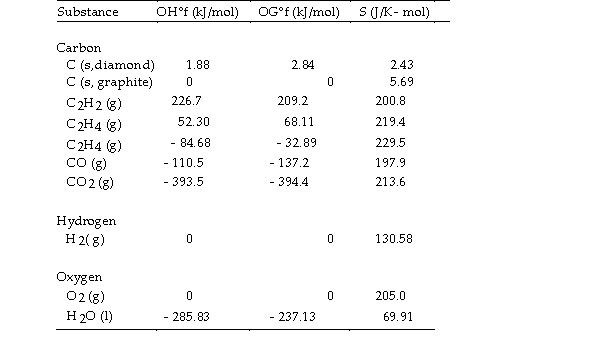 The value of ΔS° for the oxidation of carbon to carbon
The value of ΔS° for the oxidation of carbon to carbon  Is J/K. The combustion of carbon, as in charcoal briquettes, in the presence of abundant oxygen produces carbon dioxide.
Is J/K. The combustion of carbon, as in charcoal briquettes, in the presence of abundant oxygen produces carbon dioxide.
(Multiple Choice)
4.9/5  (32)
(32)
Find the temperature above which a reaction with a OH of 123 .0 kJ/mol and a OS of 90.00 J/K·mol becomes spontaneous.
(Short Answer)
4.7/5  (29)
(29)
The standard Gibbs free energy of formation of is zero.
(a) H2O (l)
(b) Na (s)
(c) H2 (g)
(Multiple Choice)
4.8/5  (49)
(49)
Showing 1 - 20 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)