Deck 15: Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/58
Play
Full screen (f)
Deck 15: Chemical Equilibrium
1
Nitrosyl bromide decomposes according to the following equation. 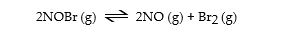
A sample of NOBr (0.64 mol) was placed in a 1.00- L flask containing no NO or Br2. At equilibrium the flask contained 0.46 mol of NOBr. How many moles of NO and Br2, respectively, are in the flask at equilibrium?
A) 0.46, 0.23
B) 0.46, 0.46
C) 0.18, 0.360
D) 0.18, 0.18
E) 0.18, 0.090
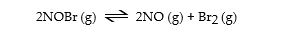
A sample of NOBr (0.64 mol) was placed in a 1.00- L flask containing no NO or Br2. At equilibrium the flask contained 0.46 mol of NOBr. How many moles of NO and Br2, respectively, are in the flask at equilibrium?
A) 0.46, 0.23
B) 0.46, 0.46
C) 0.18, 0.360
D) 0.18, 0.18
E) 0.18, 0.090
0.18, 0.090
2
How is the reaction quotient used to determine whether a system is at equilibrium?
A)The reaction is at equilibrium when Q > Keq.
B)The reaction is at equilibrium when Q = Keq.
C)The reaction quotient must be satisfied for equilibrium to be achieved.
D)The reaction is at equilibrium when Q < Keq.
E)At equilibrium, the reaction quotient is undefined.
A)The reaction is at equilibrium when Q > Keq.
B)The reaction is at equilibrium when Q = Keq.
C)The reaction quotient must be satisfied for equilibrium to be achieved.
D)The reaction is at equilibrium when Q < Keq.
E)At equilibrium, the reaction quotient is undefined.
The reaction is at equilibrium when Q = Keq.
3
What role did Karl Bosch play in development of the Haber- Bosch process?
A)He originally isolated ammonia from camel dung and found a method for purifying it.
B)He was the German industrialist who financed the research done by Haber.
C)He discovered the reaction conditions necessary for formation of ammonia.
D)He developed the equipment necessary for industrial production of ammonia.
E)Haber was working in his lab with his instructor at the time he worked out the process.
A)He originally isolated ammonia from camel dung and found a method for purifying it.
B)He was the German industrialist who financed the research done by Haber.
C)He discovered the reaction conditions necessary for formation of ammonia.
D)He developed the equipment necessary for industrial production of ammonia.
E)Haber was working in his lab with his instructor at the time he worked out the process.
He developed the equipment necessary for industrial production of ammonia.
4
The equilibrium- constant expression depends on the of the reaction.
A)the quantities of reactants and products initially present
B)stoichiometry and mechanism
C)stoichiometry
D)mechanism
E)temperature
A)the quantities of reactants and products initially present
B)stoichiometry and mechanism
C)stoichiometry
D)mechanism
E)temperature

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
5
The equilibrium- constant expression for the reaction
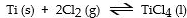
is given by
A)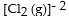
B)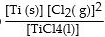
C)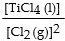
D)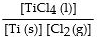
E)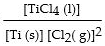
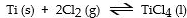
is given by
A)
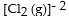
B)
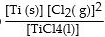
C)
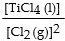
D)
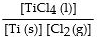
E)
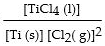

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
6
At 900 K, the equilibrium constant (Kp) for the following reaction is 0.345. 2SO2 + O2 (g) -2SO3 (g)
At equilibrium, the partial pressure of SO2 is 35.0 atm and that of O2 is 15.9 atm. The partial pressure of SO3 is _ atm.
A) 6.20 × 10- 4
B) 192
C) 40.2
D) 82.0
E) 4.21 × 10- 3
At equilibrium, the partial pressure of SO2 is 35.0 atm and that of O2 is 15.9 atm. The partial pressure of SO3 is _ atm.
A) 6.20 × 10- 4
B) 192
C) 40.2
D) 82.0
E) 4.21 × 10- 3

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
7
The Keq for the equilibrium below is 7.52 × 10- 2 at 480 °C.
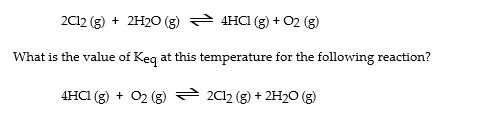
A) 5.66 × 10- 3

B) 0.150
C) 0.0752
D) - 0.0752
E) 13.3
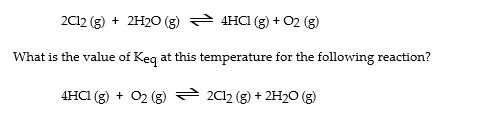
A) 5.66 × 10- 3

B) 0.150
C) 0.0752
D) - 0.0752
E) 13.3

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
8
The equilibrium constant for reaction 1 is K. The equilibrium constant for reaction 2 is . (1) SO2 (g) + (1/2) O2 (g)  SO3 (g)
SO3 (g)
(2) 2SO3 (g) 2SO2 (g) + O2 (g)
2SO2 (g) + O2 (g)
A) 1/K2
B) K2
C) 2K
D) - K2
E) 1/2K
 SO3 (g)
SO3 (g)(2) 2SO3 (g)
 2SO2 (g) + O2 (g)
2SO2 (g) + O2 (g)A) 1/K2
B) K2
C) 2K
D) - K2
E) 1/2K

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
9
The Keq for the equilibrium below is 7.52 × 10- 2 at 480 °C.
2Cl2 (g) + 2H2O (g) 4HCl (g) + O2 (g)
4HCl (g) + O2 (g)
What is the value of Keq at this temperature for the following reaction?
Cl2 (g) + H2O (g) 2HCl (g) + 1 O2 (g)
2HCl (g) + 1 O2 (g)
A) 0.274
B) 5.66 × 10- 3
C) 0.150
D) 0.0752
E) 0.0376
2Cl2 (g) + 2H2O (g)
 4HCl (g) + O2 (g)
4HCl (g) + O2 (g)What is the value of Keq at this temperature for the following reaction?
Cl2 (g) + H2O (g)
 2HCl (g) + 1 O2 (g)
2HCl (g) + 1 O2 (g)A) 0.274
B) 5.66 × 10- 3
C) 0.150
D) 0.0752
E) 0.0376

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
10
The equilibrium constant (Kp) for the reaction below is 7.00 × 10- 2 at 22 °C:  A sample of NH4HS is placed in an evacuated container and allowed to come to equilibrium. The partial pressure of NH3 is then increased by the addition of 0.590 atm of NH3. The partial pressure of H2S at equilibrium is now atm.
A sample of NH4HS is placed in an evacuated container and allowed to come to equilibrium. The partial pressure of NH3 is then increased by the addition of 0.590 atm of NH3. The partial pressure of H2S at equilibrium is now atm.
A) 0.691
B) 0.855
C) 0.119
D) 0.265
E) 0.101
 A sample of NH4HS is placed in an evacuated container and allowed to come to equilibrium. The partial pressure of NH3 is then increased by the addition of 0.590 atm of NH3. The partial pressure of H2S at equilibrium is now atm.
A sample of NH4HS is placed in an evacuated container and allowed to come to equilibrium. The partial pressure of NH3 is then increased by the addition of 0.590 atm of NH3. The partial pressure of H2S at equilibrium is now atm.A) 0.691
B) 0.855
C) 0.119
D) 0.265
E) 0.101

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
11
The equilibrium constant for the gas phase reaction 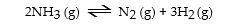
Is Keq = 230 at 300 °C. At equilibrium, _ _.
A)products predominate
B)only reactants are present
C)reactants predominate
D)only products are present
E)roughly equal amounts of products and reactants are present
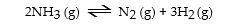
Is Keq = 230 at 300 °C. At equilibrium, _ _.
A)products predominate
B)only reactants are present
C)reactants predominate
D)only products are present
E)roughly equal amounts of products and reactants are present

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
12
The relationship between the rate constants for the forward and reverse reactions and the equilibrium constant for the process is Keq= .
A)kf + kr
B) kfkr
C) kf/kr
D) kr/kf
E) kf - kr
A)kf + kr
B) kfkr
C) kf/kr
D) kr/kf
E) kf - kr

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
13
The Keq for the equilibrium below is 0.112 at 700 °C. SO2 (g) + 1 O2
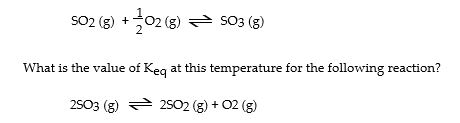
A) 79.7
B) 8.93
C) 17.86
D) 4.46
E) 2.99
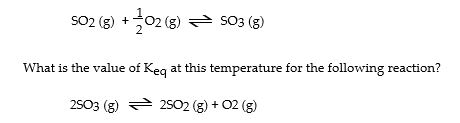
A) 79.7
B) 8.93
C) 17.86
D) 4.46
E) 2.99

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
14
In what year was Fritz Haber awarded the Nobel Prize in chemistry for his development of a process for synthesizing ammonia directly from nitrogen and hydrogen?
A) 1912
B) 1918
C) 1900
D) 1954
E) 1933
A) 1912
B) 1918
C) 1900
D) 1954
E) 1933

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
15
Consider the following reaction at equilibrium:
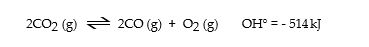
Le Cha^telier's principle predicts that adding O2 (g) to the reaction container will _ .
A)increase the partial pressure of CO (g) at equilibrium
B)increase the partial pressure of CO2 (g) at equilibrium
C)decrease the partial pressure of CO2 (g) at equilibrium
D)decrease the value of the equilibrium constant
E)increase the value of the equilibrium constant
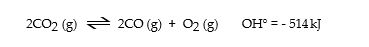
Le Cha^telier's principle predicts that adding O2 (g) to the reaction container will _ .
A)increase the partial pressure of CO (g) at equilibrium
B)increase the partial pressure of CO2 (g) at equilibrium
C)decrease the partial pressure of CO2 (g) at equilibrium
D)decrease the value of the equilibrium constant
E)increase the value of the equilibrium constant

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
16
Consider the following equilibrium.
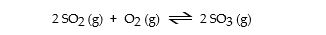
The equilibrium cannot be established when _ _ is/are placed in a 1.0- L container.
A)0.50 mol O2 (g) and 0.50 mol SO3 (g)
B)0.75 mol SO2 (g)
C)0.25 mol of SO2 (g) and 0.25 mol of SO3 (g)
D)1.0 mol SO3 (g)
E)0.25 mol SO2 (g) and 0.25 mol O2 (g)
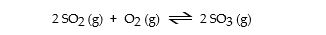
The equilibrium cannot be established when _ _ is/are placed in a 1.0- L container.
A)0.50 mol O2 (g) and 0.50 mol SO3 (g)
B)0.75 mol SO2 (g)
C)0.25 mol of SO2 (g) and 0.25 mol of SO3 (g)
D)1.0 mol SO3 (g)
E)0.25 mol SO2 (g) and 0.25 mol O2 (g)

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
17
The value of Keq for the following reaction is 0.25:

A) 0.12
B) 0.062
C) 16
D) 0.25
E) 0.50

A) 0.12
B) 0.062
C) 16
D) 0.25
E) 0.50

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
18
The Keq for the equilibrium below is 7.52 × 10- 2 at 480 °C.
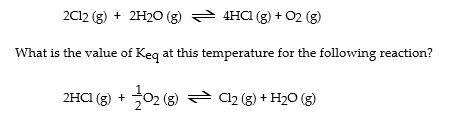
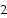
A) 0.274
B) 3.65
C) - 0.0376
D) 13.3
E) 5.66 × 10- 3
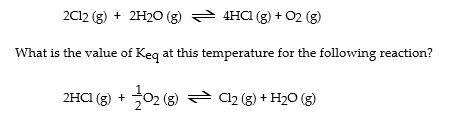
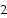
A) 0.274
B) 3.65
C) - 0.0376
D) 13.3
E) 5.66 × 10- 3

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
19
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction:
PCl3 (g) + Cl2 (g) -PCl5 (g)
An equilibrium mixture at 450 K contains PPCl3 = 0.202 atm,
PCl2 = 0.256 atm, and
PPCl5 = 3.45 atm. What is the value of Kp at this temperature?
A) 1.50 × 10- 2
B) 66.8
C) 2.99
D) 7.54
E) 1.78 × 10- 1
PCl3 (g) + Cl2 (g) -PCl5 (g)
An equilibrium mixture at 450 K contains PPCl3 = 0.202 atm,
PCl2 = 0.256 atm, and
PPCl5 = 3.45 atm. What is the value of Kp at this temperature?
A) 1.50 × 10- 2
B) 66.8
C) 2.99
D) 7.54
E) 1.78 × 10- 1

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
20
How does the reaction quotient of a reaction (Q) differ from the equilibrium constant (Keq) of the same reaction?
A)K does not depend on the concentrations or partial pressures of reaction components.
B)Q does not change with temperature.
C)Q is the same as Keq when a reaction is at equilibrium.
D)Keq does not change with temperature, whereas Q is temperature dependent.
E)Q does not depend on the concentrations or partial pressures of reaction components.
A)K does not depend on the concentrations or partial pressures of reaction components.
B)Q does not change with temperature.
C)Q is the same as Keq when a reaction is at equilibrium.
D)Keq does not change with temperature, whereas Q is temperature dependent.
E)Q does not depend on the concentrations or partial pressures of reaction components.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
21
For the endothermic reaction
CaCO3 (s) CaO (s) + CO2 (g)
CaO (s) + CO2 (g)
Le Cha^telier's principle predicts that _ will result in an increase in the number of moles of CO2.
A)increasing the pressure
B)decreasing the temperature
C)increasing the temperature
D)removing some of the CaCO3 (s)
E)adding more CaCO3 (s)
CaCO3 (s)
 CaO (s) + CO2 (g)
CaO (s) + CO2 (g)Le Cha^telier's principle predicts that _ will result in an increase in the number of moles of CO2.
A)increasing the pressure
B)decreasing the temperature
C)increasing the temperature
D)removing some of the CaCO3 (s)
E)adding more CaCO3 (s)

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
22
The effect of a catalyst on an equilibrium is to .
A)slow the reverse reaction only
B)shift the equilibrium to the right
C)increase the rate of the forward reaction only
D)increase the rate at which equilibrium is achieved without changing the composition of the equilibrium mixture
E)increase the equilibrium constant so that products are favored
A)slow the reverse reaction only
B)shift the equilibrium to the right
C)increase the rate of the forward reaction only
D)increase the rate at which equilibrium is achieved without changing the composition of the equilibrium mixture
E)increase the equilibrium constant so that products are favored

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
23
At 400 K, the equilibrium constant for the reaction Br2 (g) + Cl2 (g)  2BrCl (g)
2BrCl (g)
Is Kp = 7.0. A closed vessel at 400 K is charged with 1.00 atm of Br2 (g), 1.00 atm of Cl2 (g), and 2.00
Atm of BrCl (g). Use Q to determine which of the statements below is true.
A)At equilibrium, the total pressure in the vessel will be less than the initial total pressure.
B)The equilibrium partial pressures of Br2, Cl2, and BrCl will be the same as the initial values.
C)The equilibrium partial pressure of BrCl (g) will be greater than 2.00 atm.
D)The reaction will go to completion since there are equal amounts of Br2 and Cl2.
E)The equilibrium partial pressure of Br2 will be greater than 1.00 atm.
 2BrCl (g)
2BrCl (g)Is Kp = 7.0. A closed vessel at 400 K is charged with 1.00 atm of Br2 (g), 1.00 atm of Cl2 (g), and 2.00
Atm of BrCl (g). Use Q to determine which of the statements below is true.
A)At equilibrium, the total pressure in the vessel will be less than the initial total pressure.
B)The equilibrium partial pressures of Br2, Cl2, and BrCl will be the same as the initial values.
C)The equilibrium partial pressure of BrCl (g) will be greater than 2.00 atm.
D)The reaction will go to completion since there are equal amounts of Br2 and Cl2.
E)The equilibrium partial pressure of Br2 will be greater than 1.00 atm.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
24
The Kp for the reaction below is 1.49 × 108 at 100 °C: CO (g) + Cl2 (g) -COCl2 (g)
In an equilibrium mixture of the three gases, PCO = PCl2 = 8.60 × 10- 4 atm. The partial pressure of the product, phosgene (COCl2), is atm.
A) 1.28 × 105
B) 1.72 × 1011
C) 1.10 × 102
D) 4.96 × 10- 15
E) 2.01 × 1014
In an equilibrium mixture of the three gases, PCO = PCl2 = 8.60 × 10- 4 atm. The partial pressure of the product, phosgene (COCl2), is atm.
A) 1.28 × 105
B) 1.72 × 1011
C) 1.10 × 102
D) 4.96 × 10- 15
E) 2.01 × 1014

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
25
The number obtained by substituting starting reactant and product concentrations into an equilibrium- constant expression is known as the .

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
26
Pure and pure are excluded from equilibrium- constant expressions.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
27
Dinitrogentetraoxide partially decomposes according to the following equilibrium:
N2O4 (g) -2NO2 (g)
A 1)000- L flask is charged with 3.00 × 10- 2 mol of N2O4. At equilibrium, 2.36 × 10- 2 mol of N2O4 remains. Keq for this reaction is .
A) 0.391
B) 0.723
C) 6.93 × 10- 3
D) 1.92 × 10- 4
E) 0.212
N2O4 (g) -2NO2 (g)
A 1)000- L flask is charged with 3.00 × 10- 2 mol of N2O4. At equilibrium, 2.36 × 10- 2 mol of N2O4 remains. Keq for this reaction is .
A) 0.391
B) 0.723
C) 6.93 × 10- 3
D) 1.92 × 10- 4
E) 0.212

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
28
In which of the following reactions would increasing pressure at constant temperature not change the concentrations of reactants and products, based on Le Cha^telier's principle?
A)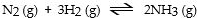
B)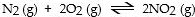
C)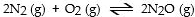
D)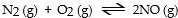
E)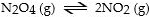
A)
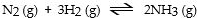
B)
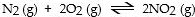
C)
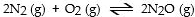
D)
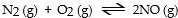
E)
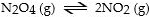

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
29
At equilibrium, .
A)all chemical reactions have ceased
B)the rate constants of the forward and reverse reactions are equal
C)the rates of the forward and reverse reactions are equal
D)the value of the equilibrium constant is 1
E)the limiting reagent has been consumed
A)all chemical reactions have ceased
B)the rate constants of the forward and reverse reactions are equal
C)the rates of the forward and reverse reactions are equal
D)the value of the equilibrium constant is 1
E)the limiting reagent has been consumed

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
30
Which one of the following is true concerning the Haber process?
A)It is a process used for the synthesis of ammonia.
B)It is an industrial synthesis of sodium chloride that was discovered by Karl Haber.
C)It is a process used for shifting equilibrium positions to the right for more economical chemical synthesis of a variety of substances.
D)It is another way of stating LeChatelier's principle.
E)It is a process for the synthesis of elemental chlorine.
A)It is a process used for the synthesis of ammonia.
B)It is an industrial synthesis of sodium chloride that was discovered by Karl Haber.
C)It is a process used for shifting equilibrium positions to the right for more economical chemical synthesis of a variety of substances.
D)It is another way of stating LeChatelier's principle.
E)It is a process for the synthesis of elemental chlorine.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
31
The equilibrium constant for the gas phase reaction N2 (g) + 3H2 (g)  2NH3 (g)
2NH3 (g)
Is Keq = 4.34 × 10- 3 at 300 °C. At equilibrium, _ _.
A)roughly equal amounts of products and reactants are present
B)reactants predominate
C)only reactants are present
D)products predominate
E)only products are present
 2NH3 (g)
2NH3 (g)Is Keq = 4.34 × 10- 3 at 300 °C. At equilibrium, _ _.
A)roughly equal amounts of products and reactants are present
B)reactants predominate
C)only reactants are present
D)products predominate
E)only products are present

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
32
Consider the following reaction at equilibrium:
2NH3 (g) N2 (g) + 3H2 (g) OH° = +92.4 kJ
N2 (g) + 3H2 (g) OH° = +92.4 kJ
Le Cha^telier's principle predicts that adding N2 (g) to the system at equilibrium will result in
A)a decrease in the concentration of NH3 (g)
B)removal of all of the H2 (g)
C)a lower partial pressure of N2
D)an increase in the value of the equilibrium constant
E)a decrease in the concentration of H2 (g)
2NH3 (g)
 N2 (g) + 3H2 (g) OH° = +92.4 kJ
N2 (g) + 3H2 (g) OH° = +92.4 kJLe Cha^telier's principle predicts that adding N2 (g) to the system at equilibrium will result in
A)a decrease in the concentration of NH3 (g)
B)removal of all of the H2 (g)
C)a lower partial pressure of N2
D)an increase in the value of the equilibrium constant
E)a decrease in the concentration of H2 (g)

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
33
Which one of the following will change the value of an equilibrium constant?
A)adding other substances that do not react with any of the species involved in the equilibrium
B)varying the initial concentrations of products
C)changing temperature
D)varying the initial concentrations of reactants
E)changing the volume of the reaction vessel
A)adding other substances that do not react with any of the species involved in the equilibrium
B)varying the initial concentrations of products
C)changing temperature
D)varying the initial concentrations of reactants
E)changing the volume of the reaction vessel

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the following reaction at equilibrium:
2CO2 (g) 2CO (g) + O2 (g) OH° = - 514 kJ
2CO (g) + O2 (g) OH° = - 514 kJ
Le Cha^telier's principle predicts that an increase in temperature will .
A)increase the value of the equilibrium constant
B)decrease the partial pressure of CO2 (g)
C)decrease the value of the equilibrium constant
D)increase the partial pressure of O2 (g)
E)increase the partial pressure of CO
2CO2 (g)
 2CO (g) + O2 (g) OH° = - 514 kJ
2CO (g) + O2 (g) OH° = - 514 kJLe Cha^telier's principle predicts that an increase in temperature will .
A)increase the value of the equilibrium constant
B)decrease the partial pressure of CO2 (g)
C)decrease the value of the equilibrium constant
D)increase the partial pressure of O2 (g)
E)increase the partial pressure of CO

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
35
If the value for the equilibrium constant is much greater than 1, then the equilibrium mixture contains mostly .

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
36
The reaction below is exothermiC: 2SO2 (g) + O2 (g)  2SO3 (g)
2SO3 (g)
Le Cha^telier's Principle predicts that will result in an increase in the number of moles of
SO3 (g) in the reaction container.
A)removing some oxygen
B)increasing the volume of the container
C)decreasing the pressure
D)increasing the pressure
E)increasing the temperature
 2SO3 (g)
2SO3 (g)Le Cha^telier's Principle predicts that will result in an increase in the number of moles of
SO3 (g) in the reaction container.
A)removing some oxygen
B)increasing the volume of the container
C)decreasing the pressure
D)increasing the pressure
E)increasing the temperature

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following reaction at equilibrium.
2CO2 (g) 2CO (g) + O2 (g) OH° = - 514 kJ
2CO (g) + O2 (g) OH° = - 514 kJ
Le Cha^telier's principle predicts that the equilibrium partial pressure of CO (g) can be maximized by carrying out the reaction .
A)at high temperature and high pressure
B)at high temperature and low pressure
C)at low temperature and low pressure
D)at low temperature and high pressure
E)in the presence of solid carbon
2CO2 (g)
 2CO (g) + O2 (g) OH° = - 514 kJ
2CO (g) + O2 (g) OH° = - 514 kJLe Cha^telier's principle predicts that the equilibrium partial pressure of CO (g) can be maximized by carrying out the reaction .
A)at high temperature and high pressure
B)at high temperature and low pressure
C)at low temperature and low pressure
D)at low temperature and high pressure
E)in the presence of solid carbon

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the following reaction at equilibrium:
2SO2 (g) + O2 (g) 2SO3 (g) OH° = - 99 kJ
2SO3 (g) OH° = - 99 kJ
Le Cha^telier's principle predicts that an increase in temperature will result in .
A)the partial pressure of O2 will decrease
B)a decrease in the partial pressure of SO3
C)a decrease in the partial pressure of SO2
D)an increase in Keq
E)no changes in equilibrium partial pressures
2SO2 (g) + O2 (g)
 2SO3 (g) OH° = - 99 kJ
2SO3 (g) OH° = - 99 kJLe Cha^telier's principle predicts that an increase in temperature will result in .
A)the partial pressure of O2 will decrease
B)a decrease in the partial pressure of SO3
C)a decrease in the partial pressure of SO2
D)an increase in Keq
E)no changes in equilibrium partial pressures

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
39
Exactly 3.5 moles if N2O4 is placed in an empty 2.0- L container and allowed to reach equilibrium described by the equation
N2O4 (g) "2NO2 (g)
If at equilibrium the N2O4 is 25% dissociated, what is the value of the equilibrium constant for the reaction?
N2O4 (g) "2NO2 (g)
If at equilibrium the N2O4 is 25% dissociated, what is the value of the equilibrium constant for the reaction?

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
40
The equilibrium- constant expression for a reaction written in one direction is the
of the one for the reaction written for the reverse direction.
of the one for the reaction written for the reverse direction.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
41
Dinitrogentetraoxide partially decomposes according to the following equilibrium:
N2O4 (g) 2NO2 (g)
2NO2 (g)
A 1)00- L flask is charged with 0.400 mol of N2O4. At equilibrium at 373 K, 0.0055 mol of N2O4 remains. Keq for this reaction is .
A) 2.2 × 10- 4
B) 0.22
C) 0.87
D) 13
E) 0.022
N2O4 (g)
 2NO2 (g)
2NO2 (g)A 1)00- L flask is charged with 0.400 mol of N2O4. At equilibrium at 373 K, 0.0055 mol of N2O4 remains. Keq for this reaction is .
A) 2.2 × 10- 4
B) 0.22
C) 0.87
D) 13
E) 0.022

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
42
The effect of a catalyst on a chemical reaction is to react with product, effectively removing it and shifting the equilibrium to the right.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
43
The relationship between the concentrations of reactants and products of a system at equilibrium is given by the law of mass action.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
44
At elevated temperatures, molecular hydrogen and molecular bromine react to partially form hydrogen bromide:
H2 (g) + Br2 (g) 2HBr (g)
2HBr (g)
A mixture of 0.682 mol of H2 and 0.440 mol of Br2 is combined in a reaction vessel with a volume of 2.00 L. At equilibrium at 700 K, there are 0.566 mol of H2 present. At equilibrium, there are
Mol of Br2 present in the reaction vessel.
A) 0.440
B) 0.324
C) 0.000
D) 0.566
E) 0.232
H2 (g) + Br2 (g)
 2HBr (g)
2HBr (g)A mixture of 0.682 mol of H2 and 0.440 mol of Br2 is combined in a reaction vessel with a volume of 2.00 L. At equilibrium at 700 K, there are 0.566 mol of H2 present. At equilibrium, there are
Mol of Br2 present in the reaction vessel.
A) 0.440
B) 0.324
C) 0.000
D) 0.566
E) 0.232

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the following chemical reaction:
H2 (g) + I2 (g) 2HI (g)
2HI (g)
At equilibrium in a particular experiment, the concentrations of H2, I2, and HI were 0.15 M,
0)033 M, and 0.55 M, respectively. The value of Keq for this reaction is .
A) 9.0 × 10- 3
B) 61
C) 23
D) 111
E) 6.1
H2 (g) + I2 (g)
 2HI (g)
2HI (g)At equilibrium in a particular experiment, the concentrations of H2, I2, and HI were 0.15 M,
0)033 M, and 0.55 M, respectively. The value of Keq for this reaction is .
A) 9.0 × 10- 3
B) 61
C) 23
D) 111
E) 6.1

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
46
At 22 °C, Kp = 0.070 for the equilibrium:
NH4HS (s) NH3 (g) + H2S (g)
NH3 (g) + H2S (g)
A sample of solid NH4HS is placed in a closed vessel and allowed to equilibrate. Calculate the equilibrium partial pressure (atm) of ammonia, assuming that some solid NH4HS remains.
A) 0.070
B) 0.26
C) 3.8
D) 0.52
E) 4.9 × 10- 3
NH4HS (s)
 NH3 (g) + H2S (g)
NH3 (g) + H2S (g)A sample of solid NH4HS is placed in a closed vessel and allowed to equilibrate. Calculate the equilibrium partial pressure (atm) of ammonia, assuming that some solid NH4HS remains.
A) 0.070
B) 0.26
C) 3.8
D) 0.52
E) 4.9 × 10- 3

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
47
In an exothermic equilibrium reaction, increasing the reaction temperature favors the formation of reactants.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
48
A sealed 1.0 L flask is charged with 0.500 mol of I2 and 0.500 mol of Br2. An equilibrium reaction ensues:
I2 (g) + Br2 (g) 2IBr (g)
2IBr (g)
When the container contents achieve equilibrium, the flask contains 0.84 mol of IBr. The value of Keq is _ .
A) 4.0
B) 2.8
C) 11
D) 6.1
E) 110
I2 (g) + Br2 (g)
 2IBr (g)
2IBr (g)When the container contents achieve equilibrium, the flask contains 0.84 mol of IBr. The value of Keq is _ .
A) 4.0
B) 2.8
C) 11
D) 6.1
E) 110

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
49
Acetic acid is a weak acid that dissociates into the acetate ion and a proton in aqueous solution:
HC2H3O2 (aq)![<strong>Acetic acid is a weak acid that dissociates into the acetate ion and a proton in aqueous solution: HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sub> </sub>(aq) C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>- </sup>(aq) + H<sup>+</sup><sup> </sup>(aq) At equilibrium at 25 °C a 0.100 M solution of acetic acid has the following concentrations: [HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>] = 0.0990 M, [C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>-</sup><sup> </sup>] = 1.33 × 10<sup>-</sup><sup> </sup><sup>3</sup><sup> </sup>M, and [H<sup>+</sup>] = 1.33 × 10<sup>-</sup><sup> </sup><sup>3</sup><sup> </sup>M. The equilibrium Constant, K<sub>eq</sub>, for the ionization of acetic acid at 25 °C is _ .</strong> A) 5.71 × 10<sup>6</sup> B) 1.75 × 10<sup>-</sup><sup> </sup><sup>7</sup> C) 1.79 × 10<sup>-</sup><sup> </sup><sup>5</sup> D) 5.71 × 10<sup>4</sup> E) 0.100](https://storage.examlex.com/TB1819/11ead62c_1f5a_a252_ac95_795bbf0e8345_TB1819_11.jpg) C2H3O2- (aq) + H+ (aq)
C2H3O2- (aq) + H+ (aq)
At equilibrium at 25 °C a 0.100 M solution of acetic acid has the following concentrations:
[HC2H3O2] = 0.0990 M, [C2H3O2- ] = 1.33 × 10- 3 M, and [H+] = 1.33 × 10- 3 M. The equilibrium
Constant, Keq, for the ionization of acetic acid at 25 °C is _ .
A) 5.71 × 106
B) 1.75 × 10- 7
C) 1.79 × 10- 5
D) 5.71 × 104
E) 0.100
HC2H3O2 (aq)
![<strong>Acetic acid is a weak acid that dissociates into the acetate ion and a proton in aqueous solution: HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sub> </sub>(aq) C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>- </sup>(aq) + H<sup>+</sup><sup> </sup>(aq) At equilibrium at 25 °C a 0.100 M solution of acetic acid has the following concentrations: [HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub>] = 0.0990 M, [C<sub>2</sub>H<sub>3</sub>O<sub>2</sub><sup>-</sup><sup> </sup>] = 1.33 × 10<sup>-</sup><sup> </sup><sup>3</sup><sup> </sup>M, and [H<sup>+</sup>] = 1.33 × 10<sup>-</sup><sup> </sup><sup>3</sup><sup> </sup>M. The equilibrium Constant, K<sub>eq</sub>, for the ionization of acetic acid at 25 °C is _ .</strong> A) 5.71 × 10<sup>6</sup> B) 1.75 × 10<sup>-</sup><sup> </sup><sup>7</sup> C) 1.79 × 10<sup>-</sup><sup> </sup><sup>5</sup> D) 5.71 × 10<sup>4</sup> E) 0.100](https://storage.examlex.com/TB1819/11ead62c_1f5a_a252_ac95_795bbf0e8345_TB1819_11.jpg) C2H3O2- (aq) + H+ (aq)
C2H3O2- (aq) + H+ (aq)At equilibrium at 25 °C a 0.100 M solution of acetic acid has the following concentrations:
[HC2H3O2] = 0.0990 M, [C2H3O2- ] = 1.33 × 10- 3 M, and [H+] = 1.33 × 10- 3 M. The equilibrium
Constant, Keq, for the ionization of acetic acid at 25 °C is _ .
A) 5.71 × 106
B) 1.75 × 10- 7
C) 1.79 × 10- 5
D) 5.71 × 104
E) 0.100

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
50
The value of Keq for the equilibrium
H2 (g) + I2 (g) 2 HI (g)
2 HI (g)
Is 794 at 25 °C. At this temperature, what is the value of Keq for the equilibrium below?
HI (g) 1/2 H2 (g) + 1/2 I2 (g)

A) 0.035
B) 0.0013
C) 1588
D) 397
E) 28
H2 (g) + I2 (g)
 2 HI (g)
2 HI (g)Is 794 at 25 °C. At this temperature, what is the value of Keq for the equilibrium below?
HI (g) 1/2 H2 (g) + 1/2 I2 (g)

A) 0.035
B) 0.0013
C) 1588
D) 397
E) 28

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
51
The equilibrium constant (Kp) for the interconversion of PCl5 and PCl3 is 0.0121:
PCl5 (g) PCl3 (g) + Cl2 (g)
PCl3 (g) + Cl2 (g)
A vessel is charged with PCl5, giving an initial pressure of 0.123 atm. At equilibrium, the partial Pressure of PCl3 is _ atm.
A) 0.123
B) 0.078
C) 0.033
D) 0.045
E) 0.090
PCl5 (g)
 PCl3 (g) + Cl2 (g)
PCl3 (g) + Cl2 (g)A vessel is charged with PCl5, giving an initial pressure of 0.123 atm. At equilibrium, the partial Pressure of PCl3 is _ atm.
A) 0.123
B) 0.078
C) 0.033
D) 0.045
E) 0.090

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
52
The value of Keq for the equilibrium
H2 (g) + I2 (g) 2 HI (g)
2 HI (g)
Is 794 at 25 °C. What is the value of Keq for the equilibrium below?
1/2 H2 (g) + 1/2 I2 (g) HI (g)

A) 397
B) 0.035
C) 1588
D) 28
E) 0.0013
H2 (g) + I2 (g)
 2 HI (g)
2 HI (g)Is 794 at 25 °C. What is the value of Keq for the equilibrium below?
1/2 H2 (g) + 1/2 I2 (g) HI (g)

A) 397
B) 0.035
C) 1588
D) 28
E) 0.0013

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
53
Le Chatelier's principle states that if a system at equilibrium is disturbed, the equilibrium will shift to minimize the disturbance.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
54
At 200 °C, the equilibrium constant (Kp) for the reaction below is 2.40 × 103.
2NO (g) N2 (g) + O2 (g)
N2 (g) + O2 (g)
A closed vessel is charged with 36.1 atm of NO. At equilibrium, the partial pressure of O2 is
Atm)
A) 294
B) 6.00
C) 18.1
D) 35.7
E) 1.50 × 10- 2
2NO (g)
 N2 (g) + O2 (g)
N2 (g) + O2 (g)A closed vessel is charged with 36.1 atm of NO. At equilibrium, the partial pressure of O2 is
Atm)
A) 294
B) 6.00
C) 18.1
D) 35.7
E) 1.50 × 10- 2

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
55
A reaction vessel is charged with hydrogen iodide, which partially decomposes to molecular hydrogen and iodine:
2HI (g) H2(g) + I2(g)
H2(g) + I2(g)
When the system comes to equilibrium at 425 °C, PHI = 0.708 atm, and PH2 = PI2
= 0)0960 atm. The value of Kp at this temperature is .
A) 1.30 × 10- 2
B) 6.80 × 10- 2
C) Kp cannot be calculated for this gas reaction when the volume of the reaction vessel is not given.
D) 54.3
E) 1.84 × 10- 2
2HI (g)
 H2(g) + I2(g)
H2(g) + I2(g)When the system comes to equilibrium at 425 °C, PHI = 0.708 atm, and PH2 = PI2
= 0)0960 atm. The value of Kp at this temperature is .
A) 1.30 × 10- 2
B) 6.80 × 10- 2
C) Kp cannot be calculated for this gas reaction when the volume of the reaction vessel is not given.
D) 54.3
E) 1.84 × 10- 2

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
56
In the coal- gasification process, carbon monoxide is converted to carbon dioxide via the following reaction:
CO (g) + H2O (g) CO2 (g) + H2 (g)
CO2 (g) + H2 (g)
In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00- L reaction vessel. At equilibrium, there were 0.19 mol of CO remaining. Keq at the temperature of the experiment is
)
A) 0.56
B) 0.75
C) 1.78
D) 5.47
E) 1.0
CO (g) + H2O (g)
 CO2 (g) + H2 (g)
CO2 (g) + H2 (g)In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00- L reaction vessel. At equilibrium, there were 0.19 mol of CO remaining. Keq at the temperature of the experiment is
)
A) 0.56
B) 0.75
C) 1.78
D) 5.47
E) 1.0

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
57
Kp = 0.0198 at 721 K for the reaction
2HI (g) H2 (g) + I2 (g)
H2 (g) + I2 (g)
In a particular experiment, the partial pressures of H2 and I2 at equilibrium are 0.710 and 0.888 atm, respectively. The partial pressure of HI is atm.
A) 7.87
B) 1.98
C) 0.125
D) 0.389
E) 5.64
2HI (g)
 H2 (g) + I2 (g)
H2 (g) + I2 (g)In a particular experiment, the partial pressures of H2 and I2 at equilibrium are 0.710 and 0.888 atm, respectively. The partial pressure of HI is atm.
A) 7.87
B) 1.98
C) 0.125
D) 0.389
E) 5.64

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck
58
At constant temperature, reducing the volume of a gaseous equilibrium mixture causes the reaction to shift in the direction that increases the number of moles of gas in the system.

Unlock Deck
Unlock for access to all 58 flashcards in this deck.
Unlock Deck
k this deck


