Exam 15: Chemical Equilibrium
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
The Kp for the reaction below is 1.49 × 108 at 100 °C: CO (g) + Cl2 (g) -COCl2 (g)
In an equilibrium mixture of the three gases, PCO = PCl2 = 8.60 × 10- 4 atm. The partial pressure of the product, phosgene (COCl2), is atm.
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
C
At 200 °C, the equilibrium constant (Kp) for the reaction below is 2.40 × 103.
2NO (g)  N2 (g) + O2 (g)
A closed vessel is charged with 36.1 atm of NO. At equilibrium, the partial pressure of O2 is
Atm)
N2 (g) + O2 (g)
A closed vessel is charged with 36.1 atm of NO. At equilibrium, the partial pressure of O2 is
Atm)
Free
(Multiple Choice)
4.8/5  (26)
(26)
Correct Answer:
C
In an exothermic equilibrium reaction, increasing the reaction temperature favors the formation of reactants.
Free
(True/False)
4.9/5  (32)
(32)
Correct Answer:
True
The number obtained by substituting starting reactant and product concentrations into an equilibrium- constant expression is known as the .
(Short Answer)
4.8/5  (44)
(44)
In the coal- gasification process, carbon monoxide is converted to carbon dioxide via the following reaction:
CO (g) + H2O (g)  CO2 (g) + H2 (g)
In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00- L reaction vessel. At equilibrium, there were 0.19 mol of CO remaining. Keq at the temperature of the experiment is
)
CO2 (g) + H2 (g)
In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00- L reaction vessel. At equilibrium, there were 0.19 mol of CO remaining. Keq at the temperature of the experiment is
)
(Multiple Choice)
4.9/5  (34)
(34)
The equilibrium constant for the gas phase reaction N2 (g) + 3H2 (g)  2NH3 (g)
Is Keq = 4.34 × 10- 3 at 300 °C. At equilibrium, _ _.
2NH3 (g)
Is Keq = 4.34 × 10- 3 at 300 °C. At equilibrium, _ _.
(Multiple Choice)
4.9/5  (41)
(41)
A sealed 1.0 L flask is charged with 0.500 mol of I2 and 0.500 mol of Br2. An equilibrium reaction ensues:
I2 (g) + Br2 (g)  2IBr (g)
When the container contents achieve equilibrium, the flask contains 0.84 mol of IBr. The value of Keq is _ .
2IBr (g)
When the container contents achieve equilibrium, the flask contains 0.84 mol of IBr. The value of Keq is _ .
(Multiple Choice)
4.8/5  (34)
(34)
A reaction vessel is charged with hydrogen iodide, which partially decomposes to molecular hydrogen and iodine:
2HI (g)  H2(g) + I2(g)
When the system comes to equilibrium at 425 °C, PHI = 0.708 atm, and PH2 = PI2
= 0)0960 atm. The value of Kp at this temperature is .
H2(g) + I2(g)
When the system comes to equilibrium at 425 °C, PHI = 0.708 atm, and PH2 = PI2
= 0)0960 atm. The value of Kp at this temperature is .
(Multiple Choice)
4.9/5  (36)
(36)
At elevated temperatures, molecular hydrogen and molecular bromine react to partially form hydrogen bromide:
H2 (g) + Br2 (g)  2HBr (g)
A mixture of 0.682 mol of H2 and 0.440 mol of Br2 is combined in a reaction vessel with a volume of 2.00 L. At equilibrium at 700 K, there are 0.566 mol of H2 present. At equilibrium, there are
Mol of Br2 present in the reaction vessel.
2HBr (g)
A mixture of 0.682 mol of H2 and 0.440 mol of Br2 is combined in a reaction vessel with a volume of 2.00 L. At equilibrium at 700 K, there are 0.566 mol of H2 present. At equilibrium, there are
Mol of Br2 present in the reaction vessel.
(Multiple Choice)
4.9/5  (42)
(42)
The value of Keq for the equilibrium
H2 (g) + I2 (g)  2 HI (g)
Is 794 at 25 °C. At this temperature, what is the value of Keq for the equilibrium below?
HI (g) 1/2 H2 (g) + 1/2 I2 (g)
2 HI (g)
Is 794 at 25 °C. At this temperature, what is the value of Keq for the equilibrium below?
HI (g) 1/2 H2 (g) + 1/2 I2 (g)

(Multiple Choice)
4.8/5  (35)
(35)
The equilibrium- constant expression depends on the of the reaction.
(Multiple Choice)
4.8/5  (30)
(30)
Exactly 3.5 moles if N2O4 is placed in an empty 2.0- L container and allowed to reach equilibrium described by the equation
N2O4 (g) "2NO2 (g)
If at equilibrium the N2O4 is 25% dissociated, what is the value of the equilibrium constant for the reaction?
(Short Answer)
4.8/5  (35)
(35)
For the endothermic reaction
CaCO3 (s)  CaO (s) + CO2 (g)
Le Cha^telier's principle predicts that _ will result in an increase in the number of moles of CO2.
CaO (s) + CO2 (g)
Le Cha^telier's principle predicts that _ will result in an increase in the number of moles of CO2.
(Multiple Choice)
4.8/5  (39)
(39)
Consider the following reaction at equilibrium:
2NH3 (g)  N2 (g) + 3H2 (g) OH° = +92.4 kJ
Le Cha^telier's principle predicts that adding N2 (g) to the system at equilibrium will result in
N2 (g) + 3H2 (g) OH° = +92.4 kJ
Le Cha^telier's principle predicts that adding N2 (g) to the system at equilibrium will result in
(Multiple Choice)
4.8/5  (35)
(35)
The Keq for the equilibrium below is 7.52 × 10- 2 at 480 °C.
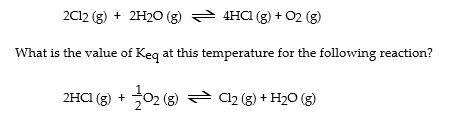
(Multiple Choice)
4.7/5  (30)
(30)
The Keq for the equilibrium below is 0.112 at 700 °C. SO2 (g) + 1 O2
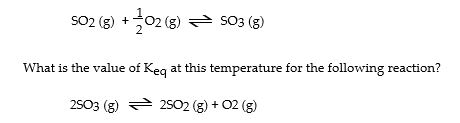
(Multiple Choice)
4.9/5  (27)
(27)
At constant temperature, reducing the volume of a gaseous equilibrium mixture causes the reaction to shift in the direction that increases the number of moles of gas in the system.
(True/False)
4.8/5  (44)
(44)
Le Chatelier's principle states that if a system at equilibrium is disturbed, the equilibrium will shift to minimize the disturbance.
(True/False)
4.7/5  (40)
(40)
In which of the following reactions would increasing pressure at constant temperature not change the concentrations of reactants and products, based on Le Cha^telier's principle?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 1 - 20 of 58
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)