Deck 7: Changes of State and Gas Laws
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 7: Changes of State and Gas Laws
1
Henry's law is 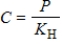 .Which statement BEST describes the meaning of this law?
.Which statement BEST describes the meaning of this law?
A) As the pressure above a gas in solution decreases, the concentration of gas in solution increases.
B) As the pressure above a gas in solution increases, the concentration of gas in solution also increases.
C) As the pressure above a gas in solution increases, the concentration of gas in solution decreases.
D) As the concentration of a gas in solution increases, Henry's constant increases.
E) As the concentration of a gas in solution decreases, Henry's constant decreases.
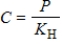 .Which statement BEST describes the meaning of this law?
.Which statement BEST describes the meaning of this law?A) As the pressure above a gas in solution decreases, the concentration of gas in solution increases.
B) As the pressure above a gas in solution increases, the concentration of gas in solution also increases.
C) As the pressure above a gas in solution increases, the concentration of gas in solution decreases.
D) As the concentration of a gas in solution increases, Henry's constant increases.
E) As the concentration of a gas in solution decreases, Henry's constant decreases.
As the pressure above a gas in solution increases, the concentration of gas in solution also increases.
2
Air is primarily composed of nitrogen (594 torr)and oxygen (160 torr).There is also carbon dioxide and water vapor in the air.Assuming that atmospheric pressure is 760 torr, what is the partial pressure of carbon dioxide and water vapor combined?
A) 1514 torr
B) 754 torr
C) 500 torr
D) 166 torr
E) 6 torr
A) 1514 torr
B) 754 torr
C) 500 torr
D) 166 torr
E) 6 torr
6 torr
3
Which statement BEST describes how the kinetic molecular view of gases can be used to explain the effect of gas temperature on gas pressure?
A) Gas pressure and gas temperature are unrelated.
B) The effect of temperature on pressure depends upon the gas.
C) As temperature increases, the particles are damaged, slowing them down and decreasing the number of collisions against the beaker; therefore the pressure decreases.
D) As temperature increases, the particles are split apart so that there are more collisions against the beaker and the pressure increases.
E) As temperature increases, the speed of particles increases, so there are more collisions against the beaker and the pressure increases.
A) Gas pressure and gas temperature are unrelated.
B) The effect of temperature on pressure depends upon the gas.
C) As temperature increases, the particles are damaged, slowing them down and decreasing the number of collisions against the beaker; therefore the pressure decreases.
D) As temperature increases, the particles are split apart so that there are more collisions against the beaker and the pressure increases.
E) As temperature increases, the speed of particles increases, so there are more collisions against the beaker and the pressure increases.
As temperature increases, the speed of particles increases, so there are more collisions against the beaker and the pressure increases.
4
Three boxes, each containing molecules in the gas phase, are illustrated below.Which box would you expect to have the highest pressure? 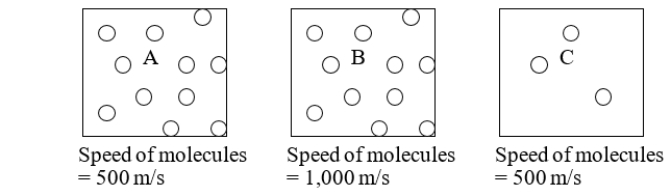
A) box A
B) box B
C) box C
D) boxes A and B have the same highest pressure.
E) boxes A and C have the same highest pressure.

A) box A
B) box B
C) box C
D) boxes A and B have the same highest pressure.
E) boxes A and C have the same highest pressure.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
This diagram shows balloons of various sizes filled with helium gas.Balloon A is at STP.If you heat balloon A, which balloon would you predict would BEST represent the new size of balloon A? 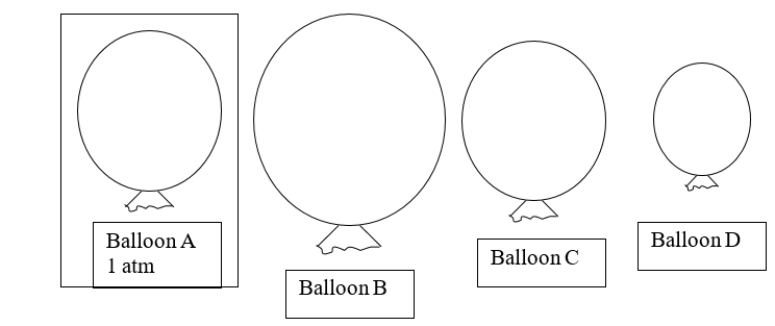
A) balloon B
B) balloon C
C) balloon D
D) either balloon B or D
E) either balloon C or D

A) balloon B
B) balloon C
C) balloon D
D) either balloon B or D
E) either balloon C or D

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following statements about pressure is FALSE?
A) Common units of pressure include atmospheres, psi, and Pa.
B) Pressure is the result of the force of atoms or molecules colliding with the walls of their container.
C) Pressure is defined as force applied to a given area.
D) Pressure increases as the number of collisions of the gas particles with their container decreases.
E) Atmospheric pressure is less than 1 atm at elevations higher than sea level.
A) Common units of pressure include atmospheres, psi, and Pa.
B) Pressure is the result of the force of atoms or molecules colliding with the walls of their container.
C) Pressure is defined as force applied to a given area.
D) Pressure increases as the number of collisions of the gas particles with their container decreases.
E) Atmospheric pressure is less than 1 atm at elevations higher than sea level.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
Which statement about the density of gases is FALSE?
A)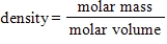
B) Gases with greater molar masses have greater densities.
C) At STP, the molar volume for gases varies widely.
D) Oxygen is denser than hydrogen.
E) A helium balloon floats in air because helium is less dense than air.
A)
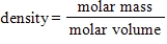
B) Gases with greater molar masses have greater densities.
C) At STP, the molar volume for gases varies widely.
D) Oxygen is denser than hydrogen.
E) A helium balloon floats in air because helium is less dense than air.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
Balloon A is placed into a container that has a higher pressure than atmospheric pressure.Which of the following statements about the relationship between pressure and volume is TRUE?
A) The pressure of the gas on the outside of the balloon forces gas inside, making balloon A bigger.
B) The pressure on the outside of the balloon will shrink the gas particles, decreasing the volume of the balloon.
C) The pressure on the outside of the balloon will slow down the gas particles, making the balloon smaller.
D) The pressure outside the balloon will compress the air in the balloon, making the balloon smaller.
E) External pressure does not affect the volume of the balloon.
A) The pressure of the gas on the outside of the balloon forces gas inside, making balloon A bigger.
B) The pressure on the outside of the balloon will shrink the gas particles, decreasing the volume of the balloon.
C) The pressure on the outside of the balloon will slow down the gas particles, making the balloon smaller.
D) The pressure outside the balloon will compress the air in the balloon, making the balloon smaller.
E) External pressure does not affect the volume of the balloon.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
Which atom has the highest electronegativity in this molecule of acetone? 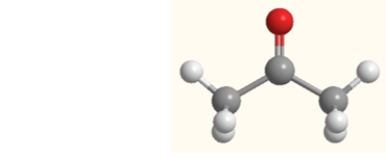
A) carbon
B) oxygen
C) hydrogen
D) carbon and hydrogen
E) They all have the same electronegativity.

A) carbon
B) oxygen
C) hydrogen
D) carbon and hydrogen
E) They all have the same electronegativity.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
You boil a pot of water (H2O)to make spaghetti and, when the water reaches its boiling point, you notice that it bubbles.What are these bubbles composed of?
A) nothing
B) H2
C) O2
D) H2 and O2
E) H2O
A) nothing
B) H2
C) O2
D) H2 and O2
E) H2O

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
One of the symptoms of the bends is joint pain.Why does joint pain occur with the bends?
A) Nitrogen bubbles expand in the joints as pressure decreases.
B) The bends causes muscle spasms, which leads to joint pain.
C) The bends causes a loss of cartilage in joints.
D) Lactic acid is released as a stress response.
E) The reason for joint pain is unknown.
A) Nitrogen bubbles expand in the joints as pressure decreases.
B) The bends causes muscle spasms, which leads to joint pain.
C) The bends causes a loss of cartilage in joints.
D) Lactic acid is released as a stress response.
E) The reason for joint pain is unknown.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following statements BEST describes the process that occurs when air is inhaled into the lungs?
A) The diaphragm moves up, the lungs expand, pressure decreases, and air is inhaled.
B) The diaphragm moves down, the lungs expand, pressure decreases, and air is inhaled.
C) The diaphragm moves up, the lungs contract, pressure increases, and air is inhaled.
D) The diaphragm moves down, the lungs expand, pressure increases, and air is inhaled.
E) The diaphragm moves down, the lungs contract, pressure decreases, and air is inhaled.
A) The diaphragm moves up, the lungs expand, pressure decreases, and air is inhaled.
B) The diaphragm moves down, the lungs expand, pressure decreases, and air is inhaled.
C) The diaphragm moves up, the lungs contract, pressure increases, and air is inhaled.
D) The diaphragm moves down, the lungs expand, pressure increases, and air is inhaled.
E) The diaphragm moves down, the lungs contract, pressure decreases, and air is inhaled.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
You have a balloon containing 1.4 L of gas in it at standard temperature and pressure.Which conversion factor would you use to convert this number to moles?
A) 1 mol/22.4 L
B) 1 L/22.4 mol
C) 22.4 L/1 mol
D) 6.02 × 1023 L/1 mol
E) 1 L/6.02 × 1023 mol
A) 1 mol/22.4 L
B) 1 L/22.4 mol
C) 22.4 L/1 mol
D) 6.02 × 1023 L/1 mol
E) 1 L/6.02 × 1023 mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
Which bond in acetone, shown below, is correctly labeled with a dipole arrow? 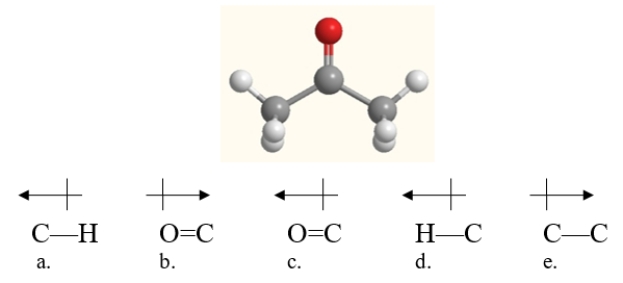
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following changes is NOT correctly labeled as a chemical reaction or a change of state?
A) rust forming on metal, a chemical reaction
B) steam coming off of a cup of coffee, a change of state
C) an ice cube melting in a drink, a change of state
D) the combustion of gasoline to give carbon dioxide and water, a chemical reaction
E) bubbles forming in a just-opened can of soda, a chemical reaction
A) rust forming on metal, a chemical reaction
B) steam coming off of a cup of coffee, a change of state
C) an ice cube melting in a drink, a change of state
D) the combustion of gasoline to give carbon dioxide and water, a chemical reaction
E) bubbles forming in a just-opened can of soda, a chemical reaction

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
How does the pressure of a gas change when it is heated in a sealed container with a constant volume? (Note: It is not a good idea to actually do this without special equipment)
A) It will not change because there is no relationship between temperature and pressure.
B) It will increase because PiTi = PfTf.
C) It will decrease because PiTi = PfTf.
D) It will increase because Pi/Ti = Pf/Tf.
E) It will decrease because Pi/Ti = Pf/Tf.
A) It will not change because there is no relationship between temperature and pressure.
B) It will increase because PiTi = PfTf.
C) It will decrease because PiTi = PfTf.
D) It will increase because Pi/Ti = Pf/Tf.
E) It will decrease because Pi/Ti = Pf/Tf.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
What is the molecular geometry of the carbon indicated by the arrow in this molecule of acetone? 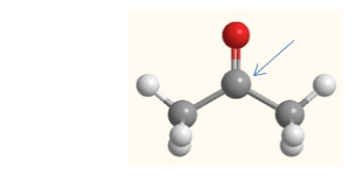
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
Imagine that you have a beaker of gas molecules as shown in the image below.If all of the gas in the beaker is transferred to a new beaker half the size of the original beaker, while maintaining the same temperature, which view BEST represents what the gas would look like? 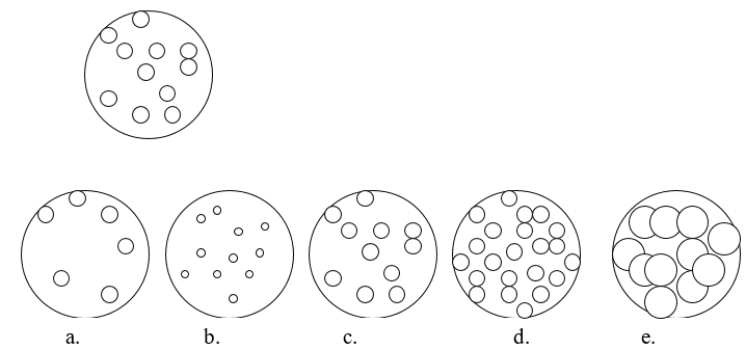
A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
At 1.0 atm, the air in a pilot's lungs occupies 0.50 L.The pilot ascends to an altitude where the pressure is 0.35 atm.If the pilot holds her breath during her ascent, what is the new volume of the air in the pilot's lungs?
A) 0.18 L
B) 0.70 L
C) 0.85 L
D) 1.4 L
E) 5.7 L
A) 0.18 L
B) 0.70 L
C) 0.85 L
D) 1.4 L
E) 5.7 L

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
In which of the following statements is the gas variable (in italics)correctly described?
A) Temperature is a measure of the space occupied by a gas.
B) Volume increases as the collisions of gas particles with the walls of the container increases.
C) Temperature is related to the average speed of gas particles.
D) The number of moles of a gas decreases as the volume of the gas increases.
E) When gas particles move faster, the pressure of the gas must increase.
A) Temperature is a measure of the space occupied by a gas.
B) Volume increases as the collisions of gas particles with the walls of the container increases.
C) Temperature is related to the average speed of gas particles.
D) The number of moles of a gas decreases as the volume of the gas increases.
E) When gas particles move faster, the pressure of the gas must increase.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
Which process requires more energy per gram: melting ice or boiling water?
A) They both require the same amount of energy because both processes involve breaking intermolecular forces.
B) Melting ice takes more energy because more intermolecular forces are broken.
C) Boiling water takes more energy because more intermolecular forces are broken.
D) Melting ice takes more energy because it occurs at 0 °C instead of 100 °C.
E) The energy of these processes has never been compared.
A) They both require the same amount of energy because both processes involve breaking intermolecular forces.
B) Melting ice takes more energy because more intermolecular forces are broken.
C) Boiling water takes more energy because more intermolecular forces are broken.
D) Melting ice takes more energy because it occurs at 0 °C instead of 100 °C.
E) The energy of these processes has never been compared.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following properties does NOT describe the kinetic-molecular view of gases?
A) Atoms and molecules in the gas phase move in straight lines and constant random motion.
B) The higher the temperature of a gas, the greater is its kinetic energy.
C) Gases are compressible.
D) Atoms and molecules are far apart from one another.
E) Dispersion forces are the only type of attractive forces found in gases.
A) Atoms and molecules in the gas phase move in straight lines and constant random motion.
B) The higher the temperature of a gas, the greater is its kinetic energy.
C) Gases are compressible.
D) Atoms and molecules are far apart from one another.
E) Dispersion forces are the only type of attractive forces found in gases.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
Atmospheric pressure at sea level is ________.At elevations higher than sea level, atmospheric pressure is_________.
A) less than 1 atm; 1 atm
B) 1 atm; less than 1 atm
C) greater than 1 atm; 1 atm
D) 1 atm; greater than 1 atm
E) 1 atm; impossible to predict
A) less than 1 atm; 1 atm
B) 1 atm; less than 1 atm
C) greater than 1 atm; 1 atm
D) 1 atm; greater than 1 atm
E) 1 atm; impossible to predict

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
What happens to acetone when it is boiled? 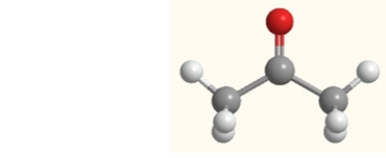
A) More intermolecular forces are formed.
B) The nonpolar covalent bonds are broken.
C) The polar covalent bonds are broken.
D) All bonds in the molecule are broken.
E) The intermolecular forces are broken.

A) More intermolecular forces are formed.
B) The nonpolar covalent bonds are broken.
C) The polar covalent bonds are broken.
D) All bonds in the molecule are broken.
E) The intermolecular forces are broken.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
Arrow B points to 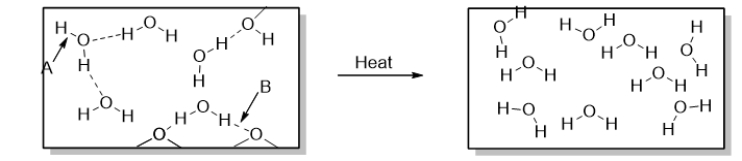
A) a polar covalent bond.
B) a nonpolar bond.
C) a dispersion force.
D) heat.
E) a hydrogen bond.

A) a polar covalent bond.
B) a nonpolar bond.
C) a dispersion force.
D) heat.
E) a hydrogen bond.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
Water has a higher heat of vaporization than carbon dioxide because water _______.
A) is a liquid at room temperature
B) freezes at a higher temperature than carbon dioxide
C) has a lower molecular weight
D) has polar covalent bonds
E) has stronger intermolecular forces of attraction.
A) is a liquid at room temperature
B) freezes at a higher temperature than carbon dioxide
C) has a lower molecular weight
D) has polar covalent bonds
E) has stronger intermolecular forces of attraction.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
Consider a warm summer's day at the beach.While the sand feels warm on your feet, the water feels cool.Which statement is the BEST explanation for this phenomenon?
A) Surface water molecules protect deeper water molecules from the Sun's rays.
B) The specific heat of water is higher than that of the sand.
C) Water is denser than sand, so it feels cooler on the skin.
D) The specific heat of sand is higher than that of water.
E) Sand is denser than water.
A) Surface water molecules protect deeper water molecules from the Sun's rays.
B) The specific heat of water is higher than that of the sand.
C) Water is denser than sand, so it feels cooler on the skin.
D) The specific heat of sand is higher than that of water.
E) Sand is denser than water.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
Which has a higher partial pressure of oxygen: inhaled air or exhaled air?
A) inhaled air, because our lungs absorb some of the oxygen in the air
B) exhaled air, because our lungs absorb some of the oxygen in the air
C) inhaled air, because oxygen is removed from the body by breathing
D) exhaled air, because oxygen is removed from the body by breathing
E) The partial pressures in inhaled and exhaled air are equal because air always contains the same concentration of oxygen.
A) inhaled air, because our lungs absorb some of the oxygen in the air
B) exhaled air, because our lungs absorb some of the oxygen in the air
C) inhaled air, because oxygen is removed from the body by breathing
D) exhaled air, because oxygen is removed from the body by breathing
E) The partial pressures in inhaled and exhaled air are equal because air always contains the same concentration of oxygen.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
A gas having a volume of 11.2 L has added to it 0.5 mol of gas at the same temperature and pressure.The final volume is 22.4 L, again at the same temperature and pressure.Which relationship is used to determine the number of moles of gas originally?
A)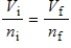
B)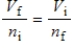
C)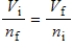
D)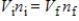
E) There is no relationship between the moles of air and the volume of air.
A)
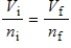
B)
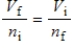
C)
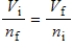
D)
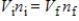
E) There is no relationship between the moles of air and the volume of air.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
The temperature of a gas at 1.00 atm and 8.00 °C is increased to 20.0 °C, resulting in a change of pressure.Which equation would you use to calculate the new pressure?
A) PiVi = PfVf
B) Pi/Vi = Pf/Vf
C) PiTi = PfTf
D) Pi/Ti = Pf/Tf
E) Vi/ni = Vf/nf
A) PiVi = PfVf
B) Pi/Vi = Pf/Vf
C) PiTi = PfTf
D) Pi/Ti = Pf/Tf
E) Vi/ni = Vf/nf

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
What happens to the speed of molecules when the molecules are frozen?
A) The speed stays the same.
B) The molecules speed up.
C) The molecules slow down.
D) The speed does not change in a predictable way.
E) The molecules stop moving completely.
A) The speed stays the same.
B) The molecules speed up.
C) The molecules slow down.
D) The speed does not change in a predictable way.
E) The molecules stop moving completely.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
Are any bonds in acetone, shown below, polar? 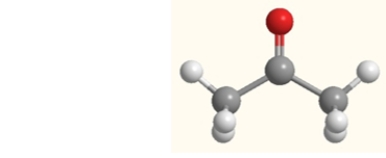
A) No, none are polar.
B) Yes, the C-C bonds are polar.
C) Yes, the C=O bond is polar.
D) Yes, the C-H bonds are polar.
E) Yes, all bonds in acetone are polar.

A) No, none are polar.
B) Yes, the C-C bonds are polar.
C) Yes, the C=O bond is polar.
D) Yes, the C-H bonds are polar.
E) Yes, all bonds in acetone are polar.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is NOT a common use of a hyperbaric oxygen therapy (HBOT)chamber?
A) treatment of diabetic wounds
B) treatment of hypertension
C) treatment of the bends
D) treatment of carbon monoxide poisoning
E) All of the above are common uses of HBOT.
A) treatment of diabetic wounds
B) treatment of hypertension
C) treatment of the bends
D) treatment of carbon monoxide poisoning
E) All of the above are common uses of HBOT.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
Which tends to be larger for a substance: the heat of vaporization or the heat of fusion?
A) They are the same because both processes involve breaking intermolecular forces.
B) The heat of vaporization is larger because more intermolecular forces are broken.
C) The heat of fusion is larger because more intermolecular forces are broken.
D) The heat of fusion is larger because melting occurs at a lower temperature.
E) The heat of vaporization is larger because boiling occurs at a high temperature.
A) They are the same because both processes involve breaking intermolecular forces.
B) The heat of vaporization is larger because more intermolecular forces are broken.
C) The heat of fusion is larger because more intermolecular forces are broken.
D) The heat of fusion is larger because melting occurs at a lower temperature.
E) The heat of vaporization is larger because boiling occurs at a high temperature.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following physical changes is the result of a decrease in temperature?
A) vaporization
B) sublimation
C) melting
D) freezing
E) All of the above are a result of a decrease in temperature.
A) vaporization
B) sublimation
C) melting
D) freezing
E) All of the above are a result of a decrease in temperature.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
What happens when a large volume of gas is compressed to a smaller volume?
A) There are more gas particles in the same volume.
B) The particles shrink.
C) The gas particles would expand in comparison to the size of the beaker.
D) Compressing the gas would likely result in gas particles lost to the atmosphere.
E) Compressing the gas is just a physical change and so should not affect how the gas particles look.
A) There are more gas particles in the same volume.
B) The particles shrink.
C) The gas particles would expand in comparison to the size of the beaker.
D) Compressing the gas would likely result in gas particles lost to the atmosphere.
E) Compressing the gas is just a physical change and so should not affect how the gas particles look.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
Does isopropyl alcohol have a higher or lower boiling point than acetone? 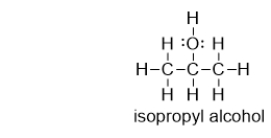
A) higher, because isopropyl alcohol can hydrogen bond
B) lower, because isopropyl alcohol can hydrogen bond
C) higher, because isopropyl alcohol can form dispersion forces
D) lower, because isopropyl alcohol can form dispersion forces
E) It is not possible to determine the answer based on the information provided.
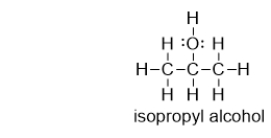
A) higher, because isopropyl alcohol can hydrogen bond
B) lower, because isopropyl alcohol can hydrogen bond
C) higher, because isopropyl alcohol can form dispersion forces
D) lower, because isopropyl alcohol can form dispersion forces
E) It is not possible to determine the answer based on the information provided.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
A sample of gaseous neon has a volume of 68.2 L at STP.How many moles of neon are in the sample?
A) 0.328 mol
B) 3.04 mol
C) 68.2 mol
D) 1530 mol
E) 4.11 × 1025 mol
A) 0.328 mol
B) 3.04 mol
C) 68.2 mol
D) 1530 mol
E) 4.11 × 1025 mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
What is the strongest intermolecular force that exists between molecules of acetone in the liquid or solid state? 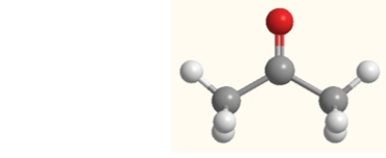
A) dispersion forces
B) dipole-dipole forces
C) hydrogen bonds
D) ionic interactions
E) None of the above forces is correct.

A) dispersion forces
B) dipole-dipole forces
C) hydrogen bonds
D) ionic interactions
E) None of the above forces is correct.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following circumstances does NOT cause decompression sickness?
A) a deep-sea diver suddenly ascending
B) flying in an airplane shortly after diving
C) hiking in the mountains
D) a miner coming out of a pressurized mine
E) cabin depressurization in an airplane
A) a deep-sea diver suddenly ascending
B) flying in an airplane shortly after diving
C) hiking in the mountains
D) a miner coming out of a pressurized mine
E) cabin depressurization in an airplane

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following processes is an exothermic physical change?
A) freezing
B) melting
C) vaporization
D) sublimation
E) All of the above are an exothermic physical change.
A) freezing
B) melting
C) vaporization
D) sublimation
E) All of the above are an exothermic physical change.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
Formaldehyde, commonly used to preserve biological specimens, is shown in the structure below.If liquid formaldehyde is boiled, which of the following illustrations BEST depicts how formaldehyde looks in the resulting vapor? 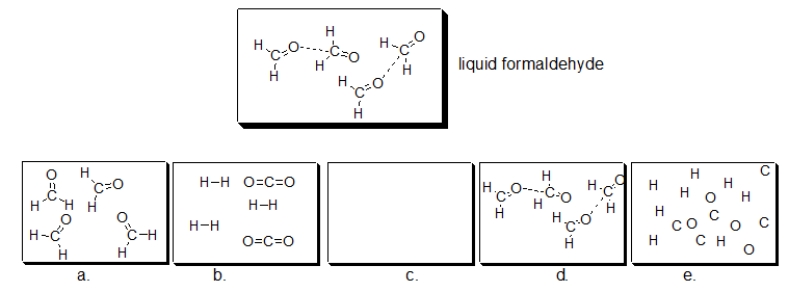
A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

A) diagram a
B) diagram b
C) diagram c
D) diagram d
E) diagram e

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
On a cool morning (12 °C), a balloon is filled with 1.5 L of helium.By mid-afternoon, the temperature has soared to 32 °C.What is the new volume of the balloon?
A) 720 L
B) 11.3 L
C) 1.6 L
D) 1.4 L
E) 0.20 L
A) 720 L
B) 11.3 L
C) 1.6 L
D) 1.4 L
E) 0.20 L

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
Henry's constant for halothane, an anesthetic, is higher than that of ether.Which anesthetic is faster acting?
A) They act at the same rates.
B) Halothane acts slower because it diffuses through tissue rather than being transported by the blood.
C) Halothane acts faster because it diffuses through tissue rather than being transported by the blood.
D) Ether acts slower because it diffuses through tissue rather than being transported by the blood.
E) Ether acts faster because it diffuses through tissue rather than being transported by the blood.
A) They act at the same rates.
B) Halothane acts slower because it diffuses through tissue rather than being transported by the blood.
C) Halothane acts faster because it diffuses through tissue rather than being transported by the blood.
D) Ether acts slower because it diffuses through tissue rather than being transported by the blood.
E) Ether acts faster because it diffuses through tissue rather than being transported by the blood.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
How are vaporization and evaporation similar?
A) Vaporization and evaporation mean exactly the same thing.
B) Both occur only at high temperatures.
C) Both occur only at very low temperatures.
D) Both always involve water.
E) Both are the transformation of liquid into a gas.
A) Vaporization and evaporation mean exactly the same thing.
B) Both occur only at high temperatures.
C) Both occur only at very low temperatures.
D) Both always involve water.
E) Both are the transformation of liquid into a gas.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
Which compound has the lowest boiling point?
A) propane, C3H8, a nonpolar covalent compound
B) ethanol, C2H6O, a polar covalent compound
C) water, H2O, a polar covalent compound
D) oxygen, O2, a nonpolar diatomic element
E) methane, CH4, a nonpolar covalent compound
A) propane, C3H8, a nonpolar covalent compound
B) ethanol, C2H6O, a polar covalent compound
C) water, H2O, a polar covalent compound
D) oxygen, O2, a nonpolar diatomic element
E) methane, CH4, a nonpolar covalent compound

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
A narrow tube on a road bike should be inflated to about 100 psi.What is this pressure in atmospheres?
A) 0.1 atm
B) 0.5 atm
C) 7 atm
D) 1,000 atm
E) 2 × 105 atm
A) 0.1 atm
B) 0.5 atm
C) 7 atm
D) 1,000 atm
E) 2 × 105 atm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following statements BEST describes specific heat?
A) It is the specific amount of heat that a substance has at any one time.
B) It is the amount of heat energy required to boil one gram of water at sea level.
C) It is the amount of heat energy actually transferred during heating.
D) It is the amount of heat energy required to melt a substance.
E) It is the amount of heat energy required to raise the temperature of one gram of a substance by one degree.
A) It is the specific amount of heat that a substance has at any one time.
B) It is the amount of heat energy required to boil one gram of water at sea level.
C) It is the amount of heat energy actually transferred during heating.
D) It is the amount of heat energy required to melt a substance.
E) It is the amount of heat energy required to raise the temperature of one gram of a substance by one degree.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
A nitrogen bubble with a volume of 0.0050 mL forms in the joint of a scuba diver as she ascends rapidly from a pressure of 4.1 atm to the surface, with a pressure of 1.0 atm.What is the volume of the bubble at the surface?
A) 820 mL
B) 4.1 mL
C) 0.0012 mL
D) 0.021 mL
E) 0.0050 mL
A) 820 mL
B) 4.1 mL
C) 0.0012 mL
D) 0.021 mL
E) 0.0050 mL

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
Two sealed boxes are filled with gas.The first box contains more molecules moving faster and therefore has a higher pressure.The second box contains fewer molecules moving slower and therefore has a lower pressure.Why does the first box have a higher pressure?
A) The more molecules, the higher the pressure.
B) The more collisions against the side of the box, the higher the pressure.
C) The fewer molecules, the higher the pressure.
D) When there is high pressure, it is harder for molecules to move, so they create heat and pressure.
E) There is no reason for the first box to have high pressure.
A) The more molecules, the higher the pressure.
B) The more collisions against the side of the box, the higher the pressure.
C) The fewer molecules, the higher the pressure.
D) When there is high pressure, it is harder for molecules to move, so they create heat and pressure.
E) There is no reason for the first box to have high pressure.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is the common treatment for the bends?
A) No treatment exists.
B) a pressure decrease to a fraction of atmospheric pressure
C) treatment with nitrogen binding drugs
D) a blood transfusion
E) slow depressurization from high pressure in a hyperbaric chamber
A) No treatment exists.
B) a pressure decrease to a fraction of atmospheric pressure
C) treatment with nitrogen binding drugs
D) a blood transfusion
E) slow depressurization from high pressure in a hyperbaric chamber

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
What type of change in pressure causes the bends, and why?
A) an increase in pressure, because nitrogen rapidly diffuses out of the bloodstream
B) an increase in pressure, because nitrogen rapidly dissolves into the bloodstream
C) a decrease in pressure, because nitrogen rapidly diffuses out of the bloodstream
D) a decrease in pressure, because nitrogen slowly dissolves into the bloodstream
E) The bends is not a result of pressure changes.
A) an increase in pressure, because nitrogen rapidly diffuses out of the bloodstream
B) an increase in pressure, because nitrogen rapidly dissolves into the bloodstream
C) a decrease in pressure, because nitrogen rapidly diffuses out of the bloodstream
D) a decrease in pressure, because nitrogen slowly dissolves into the bloodstream
E) The bends is not a result of pressure changes.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
A balloon at STP is heated.Which of the following statements about the relationship between volume and temperature is TRUE?
A) As temperature increases, the volume of a gas decreases.
B) The volume of a gas is unaffected by temperature changes.
C) The volume of a gas is affected by temperature changes, but it is not possible to predict how it is affected.
D) Increasing the temperature would make some of the gas escape from the balloon, decreasing the volume.
E) As temperature increases, the volume of a gas also increases.
A) As temperature increases, the volume of a gas decreases.
B) The volume of a gas is unaffected by temperature changes.
C) The volume of a gas is affected by temperature changes, but it is not possible to predict how it is affected.
D) Increasing the temperature would make some of the gas escape from the balloon, decreasing the volume.
E) As temperature increases, the volume of a gas also increases.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
What change of phase is represented by B on the heating curve? 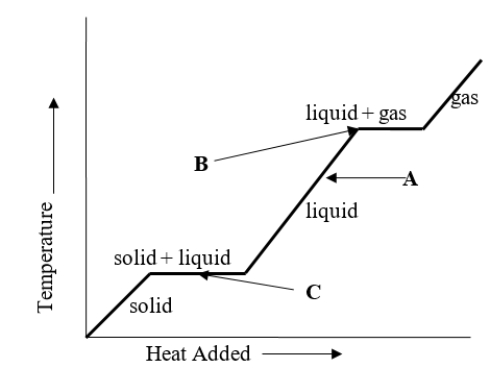
A) boiling
B) freezing
C) sublimating
D) melting
E) It is not possible to determine the change of phase at B by looking at the chart.

A) boiling
B) freezing
C) sublimating
D) melting
E) It is not possible to determine the change of phase at B by looking at the chart.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
What occurs over the course of the physical change illustrated below? 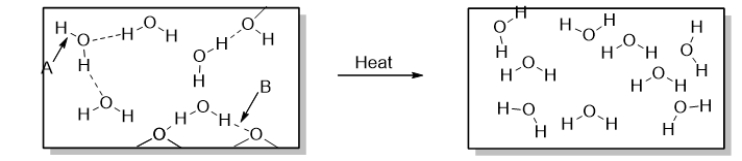
A) Hydrogen bonds are broken.
B) Hydrogen bonds are formed.
C) Polar covalent bonds are broken.
D) Polar covalent bonds are formed.
E) Both hydrogen bonds and polar covalent bonds are broken.

A) Hydrogen bonds are broken.
B) Hydrogen bonds are formed.
C) Polar covalent bonds are broken.
D) Polar covalent bonds are formed.
E) Both hydrogen bonds and polar covalent bonds are broken.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
What is the volume of 2.01 × 10-3 mol of oxygen at STP?
A) 8.97 × 10-5 L
B) 0.00201 L
C) 2.01 × 10-3 L
D) 4.50 × 10-2 L
E) 1.11 × 104 L
A) 8.97 × 10-5 L
B) 0.00201 L
C) 2.01 × 10-3 L
D) 4.50 × 10-2 L
E) 1.11 × 104 L

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
How many calories of heat are required to raise the temperature of 15 g water (specific heat = 1.00 cal/g·°C)from 25 °C and 42 °C?
A) 380 cal
B) 630 cal
C) 260 cal
D) 2.8 cal
E) 0.88 cal
A) 380 cal
B) 630 cal
C) 260 cal
D) 2.8 cal
E) 0.88 cal

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
To accomplish the change of phase shown in the diagram below, you could 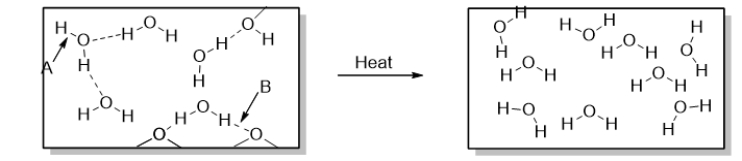
A) drink a glass of water.
B) put water in the freezer.
C) boil water.
D) let an ice cube melt in a warm glass of water.
E) let an ice cube melt in the sink.

A) drink a glass of water.
B) put water in the freezer.
C) boil water.
D) let an ice cube melt in a warm glass of water.
E) let an ice cube melt in the sink.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following statements BEST describes pressure?
A) Pressure is energy applied to a given volume.
B) Pressure is work applied to a given area.
C) Pressure is work applied to a given volume.
D) Pressure is force applied to a given area.
E) Pressure is energy, work, or force applied to a substance.
A) Pressure is energy applied to a given volume.
B) Pressure is work applied to a given area.
C) Pressure is work applied to a given volume.
D) Pressure is force applied to a given area.
E) Pressure is energy, work, or force applied to a substance.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
Why is water (H2O)a liquid at room temperature whereas methane (CH4)is a gas?
A) Water forms hydrogen bonds, and methane only forms dispersion forces.
B) Water forms dispersion forces, and methane forms hydrogen bonds.
C) Water has a higher molecular weight than methane.
D) Methane has a higher molecular weight than water.
E) Methane has more atoms than water.
A) Water forms hydrogen bonds, and methane only forms dispersion forces.
B) Water forms dispersion forces, and methane forms hydrogen bonds.
C) Water has a higher molecular weight than methane.
D) Methane has a higher molecular weight than water.
E) Methane has more atoms than water.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
A gas having a volume of 11.2 L has added to it 0.50 mol of gas at the same temperature and pressure.The final volume is 22.4 L, again at the same temperature and pressure.How many moles were there originally?
A) 0.50 mol
B) 5.6 mol
C) 0 mol
D) 2.0 mol
E) 11.2 mol
A) 0.50 mol
B) 5.6 mol
C) 0 mol
D) 2.0 mol
E) 11.2 mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
How do phase changes differ from chemical reactions?
A) There is no difference between them.
B) Phase changes occur more rapidly than chemical reactions.
C) Only chemical reactions involve changes in energy.
D) Only chemical reactions follow the conservation of matter.
E) Phase changes do not involve breaking or making covalent bonds.
A) There is no difference between them.
B) Phase changes occur more rapidly than chemical reactions.
C) Only chemical reactions involve changes in energy.
D) Only chemical reactions follow the conservation of matter.
E) Phase changes do not involve breaking or making covalent bonds.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
According to Avogadro's law, 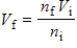 .What is the meaning of this law?
.What is the meaning of this law?
A) Adding moles of gas decreases the volume of the gas.
B) Adding moles of gas increases the volume of the gas.
C) The volume is inversely proportional to the moles of air.
D) Gas particles shrink when they are crowded.
E) Gas particles expand when they are crowded.
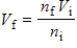 .What is the meaning of this law?
.What is the meaning of this law?A) Adding moles of gas decreases the volume of the gas.
B) Adding moles of gas increases the volume of the gas.
C) The volume is inversely proportional to the moles of air.
D) Gas particles shrink when they are crowded.
E) Gas particles expand when they are crowded.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
Because gases are compressible, they become ________ when pressure is increased.
A) very reactive
B) inert
C) solids
D) liquids
E) a lower density gas
A) very reactive
B) inert
C) solids
D) liquids
E) a lower density gas

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
Which statement about vaporization and evaporation is FALSE?
A) Vaporization is an exothermic process, and evaporation is endothermic.
B) The heat required for evaporation can come from skin, resulting in cooling.
C) The heat of vaporization is equal in magnitude to the heat of condensation.
D) The heat of vaporization is the heat that is added per gram of liquid at its boiling point for vaporization.
E) Evaporation is a change of state from liquid to gas at a temperature below the boiling point.
A) Vaporization is an exothermic process, and evaporation is endothermic.
B) The heat required for evaporation can come from skin, resulting in cooling.
C) The heat of vaporization is equal in magnitude to the heat of condensation.
D) The heat of vaporization is the heat that is added per gram of liquid at its boiling point for vaporization.
E) Evaporation is a change of state from liquid to gas at a temperature below the boiling point.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following statements BEST describes how hyperbaric oxygen therapy is used to treat carbon monoxide poisoning?
A) The oxygen replaces the carbon monoxide bound to hemoglobin.
B) The oxygen removes the carbon monoxide from the lungs.
C) The oxygen weakens the attraction of carbon monoxide to hemoglobin.
D) The oxygen immediately saturates the brain, preventing brain damage.
E) The oxygen reacts with carbon monoxide to form carbon dioxide.
A) The oxygen replaces the carbon monoxide bound to hemoglobin.
B) The oxygen removes the carbon monoxide from the lungs.
C) The oxygen weakens the attraction of carbon monoxide to hemoglobin.
D) The oxygen immediately saturates the brain, preventing brain damage.
E) The oxygen reacts with carbon monoxide to form carbon dioxide.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
At the grocery store, a bag of chips contains 0.10 L of air at 1.0 atm and 20 °C.The unopened bag of chips is taken on a camping trip in the mountains where the pressure is 0.90 atm and the temperature is 25 °C.What is the volume of air in the bag in the mountains?
A) 0.090 L
B) 0.11 L
C) 7.2 L
D) 0.14 L
E) 0.10 L
A) 0.090 L
B) 0.11 L
C) 7.2 L
D) 0.14 L
E) 0.10 L

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
In which of the following phase changes is energy transferred to a substance?
A) freezing water in the freezer
B) water condensing on the outside of a glass
C) boiling water to make steam
D) the deposition of a cloud to make snow
E) All of the above have energy transferred.
A) freezing water in the freezer
B) water condensing on the outside of a glass
C) boiling water to make steam
D) the deposition of a cloud to make snow
E) All of the above have energy transferred.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following instruments is used to measure atmospheric pressure?
A) a barometer
B) a hydrometer
C) a sphygmomanometer
D) a manometer
E) an atmospherometer
A) a barometer
B) a hydrometer
C) a sphygmomanometer
D) a manometer
E) an atmospherometer

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
In general, which is the BEST interpretation of specific heat?
A) The higher the specific heat, the less heat energy is required to increase a substance's temperature.
B) The higher the specific heat, the less heat energy is required to boil or melt a substance.
C) The higher the specific heat, the more heat energy is required to boil or melt a substance.
D) The higher the specific heat, the more heat energy is required to increase a substance's temperature.
E) The higher the specific heat, the higher the temperature of a substance.
A) The higher the specific heat, the less heat energy is required to increase a substance's temperature.
B) The higher the specific heat, the less heat energy is required to boil or melt a substance.
C) The higher the specific heat, the more heat energy is required to boil or melt a substance.
D) The higher the specific heat, the more heat energy is required to increase a substance's temperature.
E) The higher the specific heat, the higher the temperature of a substance.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
Which statement BEST describes how heat is involved in the change in the diagram below? 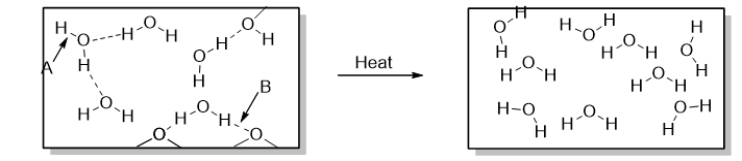
A) Heat energy breaks the intermolecular forces holding molecules of water together.
B) Heat energy is released from intermolecular forces as the molecules of water break apart.
C) Heat energy breaks the covalent bonds holding molecules of water together.
D) Heat energy is released from covalent bonds as the molecules of water break apart.
E) Heat energy is not involved in this change.

A) Heat energy breaks the intermolecular forces holding molecules of water together.
B) Heat energy is released from intermolecular forces as the molecules of water break apart.
C) Heat energy breaks the covalent bonds holding molecules of water together.
D) Heat energy is released from covalent bonds as the molecules of water break apart.
E) Heat energy is not involved in this change.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
Arrow A points to 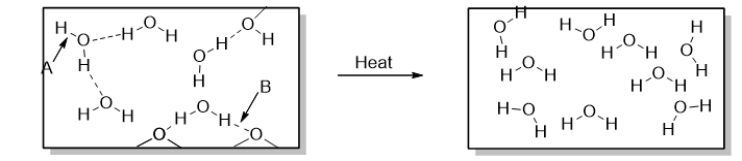
A) a polar covalent bond.
B) a nonpolar bond.
C) a dispersion force.
D) heat.
E) a hydrogen bond.

A) a polar covalent bond.
B) a nonpolar bond.
C) a dispersion force.
D) heat.
E) a hydrogen bond.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
A liquid has temperature A as shown on the heating curve.What will happen to the temperature of the liquid if heat is added to it? 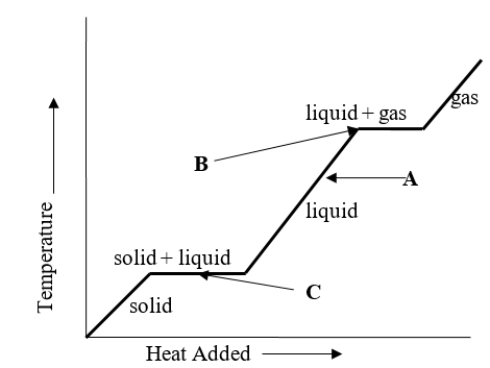
A) The temperature will stay the same.
B) The temperature will decrease.
C) The temperature will increase and then decrease.
D) The temperature will increase.
E) It is not possible to determine what will happen to the temperature of the liquid by looking at the chart.

A) The temperature will stay the same.
B) The temperature will decrease.
C) The temperature will increase and then decrease.
D) The temperature will increase.
E) It is not possible to determine what will happen to the temperature of the liquid by looking at the chart.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
This diagram shows 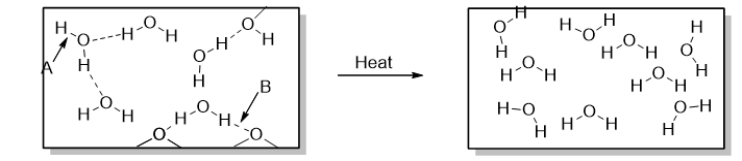
A) the decomposition of water.
B) the combustion of water.
C) solid water (ice)changing to liquid water (melting).
D) liquid water changing to water vapor (boiling).
E) water vapor changing to liquid water (condensation).

A) the decomposition of water.
B) the combustion of water.
C) solid water (ice)changing to liquid water (melting).
D) liquid water changing to water vapor (boiling).
E) water vapor changing to liquid water (condensation).

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
Which arrow BEST shows the molecular dipole of acetone? 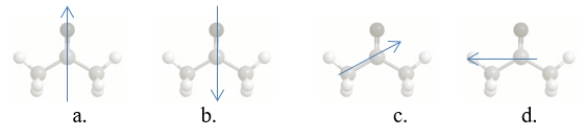
A) structure a
B) structure b
C) structure c
D) structure d
E) Acetone does not have a molecular dipole.

A) structure a
B) structure b
C) structure c
D) structure d
E) Acetone does not have a molecular dipole.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
Which statement BEST describes how heat energy is involved in changing water into steam?
A) Heat energy breaks the intermolecular forces holding molecules of water together.
B) Heat energy is released from intermolecular forces as the molecules of water break apart.
C) Heat energy breaks the covalent bonds holding molecules of water together.
D) Heat energy is released from covalent bonds as the molecules of water break apart.
E) Heat energy is not involved in this change.
A) Heat energy breaks the intermolecular forces holding molecules of water together.
B) Heat energy is released from intermolecular forces as the molecules of water break apart.
C) Heat energy breaks the covalent bonds holding molecules of water together.
D) Heat energy is released from covalent bonds as the molecules of water break apart.
E) Heat energy is not involved in this change.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
Water has a specific heat of 1.00 cal/g·°C, and wood has a specific heat of 0.10 cal/g·°C.Which substance requires more heat to be warmed from room temperature to 50 °C?
A) They both require the same amount of heat.
B) Water requires more heat because it has a higher specific heat.
C) Wood requires more heat because it has a lower specific heat.
D) Water requires more heat because it is a liquid at room temperature.
E) Wood requires more heat because it is a solid at room temperature.
A) They both require the same amount of heat.
B) Water requires more heat because it has a higher specific heat.
C) Wood requires more heat because it has a lower specific heat.
D) Water requires more heat because it is a liquid at room temperature.
E) Wood requires more heat because it is a solid at room temperature.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
The relationship between concentration and pressure in Henry's law ( 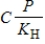 )is________.
)is________.
A) unrelated
B) exponential
C) directly proportional
D) inversely proportional
E) equal
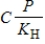 )is________.
)is________.A) unrelated
B) exponential
C) directly proportional
D) inversely proportional
E) equal

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
A scuba diver dives down to 15 m, where the pressure is 2.5 atm.The scuba diver then inhales 500.mL of air and holds his breath while ascending to the water surface, where the pressure is 1 atm.What is the volume of the air in the diver's lungs at the surface? Assume that T and n are constant.
A) 0.0050 mL
B) 0.20 mL
C) 5.0 mL
D) 200 mL
E) 1,250 mL
A) 0.0050 mL
B) 0.20 mL
C) 5.0 mL
D) 200 mL
E) 1,250 mL

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
The total pressure in a mixture of gases is equal to the partial pressure(s)of
A) the gas present in the greatest quantity.
B) all of the gases added together.
C) the gas with the lowest boiling point.
D) the gas with the highest molar mass.
E) the gas with the greatest partial pressure.
A) the gas present in the greatest quantity.
B) all of the gases added together.
C) the gas with the lowest boiling point.
D) the gas with the highest molar mass.
E) the gas with the greatest partial pressure.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


