Exam 7: Changes of State and Gas Laws
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Formaldehyde, commonly used to preserve biological specimens, is shown in the structure below.If liquid formaldehyde is boiled, which of the following illustrations BEST depicts how formaldehyde looks in the resulting vapor? 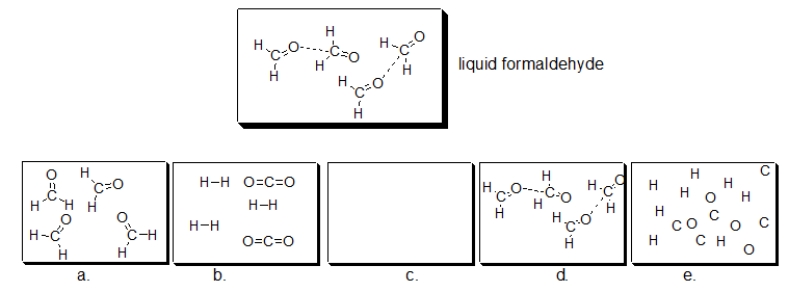
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
A
Which atom has the highest electronegativity in this molecule of acetone? 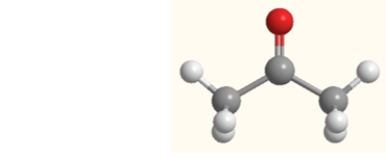
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
B
Does isopropyl alcohol have a higher or lower boiling point than acetone? 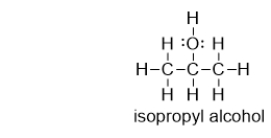
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
A
How does the pressure of a gas change when it is heated in a sealed container with a constant volume? (Note: It is not a good idea to actually do this without special equipment)
(Multiple Choice)
4.8/5  (33)
(33)
The temperature of a gas at 1.00 atm and 8.00 °C is increased to 20.0 °C, resulting in a change of pressure.Which equation would you use to calculate the new pressure?
(Multiple Choice)
5.0/5  (35)
(35)
The atmospheric pressure in Denver is 0.85 atm.What is the atmospheric pressure in torr?
(Multiple Choice)
4.8/5  (33)
(33)
In which of the following phase changes is energy transferred to a substance?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following changes of state is correctly named?
(Multiple Choice)
4.8/5  (41)
(41)
A liquid has temperature A as shown on the heating curve.What will happen to the temperature of the liquid if heat is added to it? 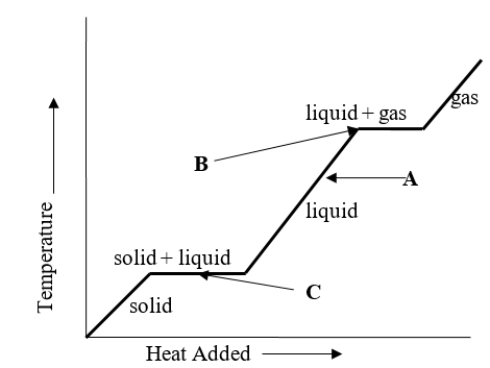
(Multiple Choice)
4.8/5  (35)
(35)
In general, which is the BEST interpretation of specific heat?
(Multiple Choice)
4.7/5  (25)
(25)
How does the pressure of a gas change when it is compressed?
(Multiple Choice)
4.8/5  (40)
(40)
Two sealed boxes are filled with gas.The first box contains more molecules moving faster and therefore has a higher pressure.The second box contains fewer molecules moving slower and therefore has a lower pressure.Why does the first box have a higher pressure?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following circumstances does NOT cause decompression sickness?
(Multiple Choice)
4.9/5  (35)
(35)
What is the molecular geometry of the carbon indicated by the arrow in this molecule of acetone? 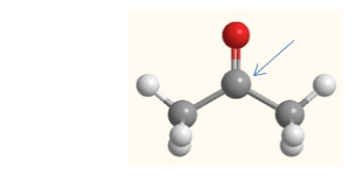
(Multiple Choice)
4.8/5  (40)
(40)
You have a 25-g sample of a metal and you would like to identify it.You are certain that the metal is either copper (specific heat = 0.093 cal/g·°C), lead (specific heat = 0.031 cal/g·°C), or aluminum (specific heat = 0.22 cal/g·°C).You run an experiment in which you find that the metal absorbs 6.2 calories of heat when it increases in temperature from 25 °C to 33 °C.Which metal is it?
(Multiple Choice)
4.8/5  (29)
(29)
Which tends to be larger for a substance: the heat of vaporization or the heat of fusion?
(Multiple Choice)
4.9/5  (39)
(39)
An oxygen tank has a pressure of 12 atm at 295 K.The maximum pressure that the tank can hold is 25 atm.What is the maximum temperature at which the tank can be stored?
(Multiple Choice)
4.7/5  (33)
(33)
Showing 1 - 20 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)