Deck 5: Chemical Quantities and Introduction to Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/88
Play
Full screen (f)
Deck 5: Chemical Quantities and Introduction to Reactions
1
When two aqueous solutions of barium chloride (BaCl2)and copper sulfate (CuSO4)are combined, insoluble barium sulfate (BaSO4)forms leaving copper chloride (CuCl2)in solution.What type of reaction is this?
A) combination
B) decomposition
C) single replacement
D) double replacement
E) oxidation-reduction
A) combination
B) decomposition
C) single replacement
D) double replacement
E) oxidation-reduction
double replacement
2
A decomposition reaction is the reverse of a _________ reaction.
A) combustion
B) combination
C) composition
D) oxidation
E) replacement
A) combustion
B) combination
C) composition
D) oxidation
E) replacement
combination
3
The only strong acid produced by the body is ______.
A) The body does not produce any strong acids.
B) H2SO4
C) HNO3
D) H3PO4
E) HCl
A) The body does not produce any strong acids.
B) H2SO4
C) HNO3
D) H3PO4
E) HCl
HCl
4
A molecule of oxygen, O2, has a molar mass of 32.00 g/mol.What is the mass of 4.52 × 1023 O2 molecules?
A) 24 g
B) 5.52 × 1023 amu
C) 5.52 × 1023 g
D) 0.75 g
E) 0.23 g
A) 24 g
B) 5.52 × 1023 amu
C) 5.52 × 1023 g
D) 0.75 g
E) 0.23 g

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements describes the processes that can occur during reduction? I.Loss of electrons
II)Gain of electrons
III)Loss of oxygens
IV)Loss of hydrogens
A) II only
B) I only
C) I and III
D) II and IV
E) II and III
II)Gain of electrons
III)Loss of oxygens
IV)Loss of hydrogens
A) II only
B) I only
C) I and III
D) II and IV
E) II and III

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
6
Pentane (C5H12)reacts with oxygen gas (O2)to form carbon dioxide (CO2)and water (H2O)according to the following reaction.What is the coefficient for oxygen in the balanced equation? C5H12 + ?O2 → ?CO2 + ?H2O
A) 2
B) 4
C) 5
D) 6
E) 8
A) 2
B) 4
C) 5
D) 6
E) 8

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
7
The balanced equation for the reaction of nitrogen and hydrogen to form ammonia is given below.What do the numbers in front of the molecules (the coefficients)mean? N2 + 3 H2 → 2 NH3
A) Three moles of H2 react with one mole of N2 to give two moles of NH3.
B) Three grams of H2 react with one gram of N2 to give two grams of NH3.
C) Three atoms of H react with one atom of N to give two molecules of NH3.
D) Two grams of N react with six grams of H to give eight grams of NH3.
E) All of the above are correct representations.
A) Three moles of H2 react with one mole of N2 to give two moles of NH3.
B) Three grams of H2 react with one gram of N2 to give two grams of NH3.
C) Three atoms of H react with one atom of N to give two molecules of NH3.
D) Two grams of N react with six grams of H to give eight grams of NH3.
E) All of the above are correct representations.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
8
What is the primary relationship between a chemical reaction and a chemical equation?
A) There is no relationship between a chemical reaction and a chemical equation.
B) A chemical equation is the written representation of a chemical reaction.
C) A chemical equation follows the law of conservation of mass but a chemical reaction does not.
D) A chemical equation shows the proportion of reactants and products but a chemical reaction does not necessarily reflect those proportions.
E) Chemical equations and chemical reactions are exactly the same thing.
A) There is no relationship between a chemical reaction and a chemical equation.
B) A chemical equation is the written representation of a chemical reaction.
C) A chemical equation follows the law of conservation of mass but a chemical reaction does not.
D) A chemical equation shows the proportion of reactants and products but a chemical reaction does not necessarily reflect those proportions.
E) Chemical equations and chemical reactions are exactly the same thing.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
9
Pentane (C5H12)reacts with oxygen gas (O2)to form carbon dioxide (CO2)and water (H2O)according to the following reaction.What is the coefficient for water in the balanced equation? C5H12 + ?O2 → ?CO2 + ?H2O
A) 2
B) 4
C) 5
D) 6
E) 8
A) 2
B) 4
C) 5
D) 6
E) 8

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
10
How many molecules of chlorine must be added to one molecule of acetylene to result in one molecule of the product shown? 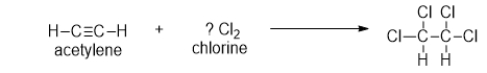
A) 0
B) 1
C) 2
D) 3
E) 4
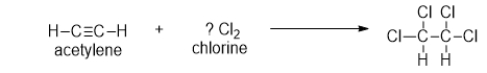
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
11
What does it mean for mass to be conserved?
A) Mass is neither created nor destroyed in the course of a chemical reaction.
B) Mass is a valuable resource and should not be wasted.
C) Molecules do not degrade under normal circumstances.
D) Atoms sometimes disappear over the course of a chemical reaction.
E) Atoms sometimes appear, disappear, or are changed over the course of a chemical reaction.
A) Mass is neither created nor destroyed in the course of a chemical reaction.
B) Mass is a valuable resource and should not be wasted.
C) Molecules do not degrade under normal circumstances.
D) Atoms sometimes disappear over the course of a chemical reaction.
E) Atoms sometimes appear, disappear, or are changed over the course of a chemical reaction.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
12
Jane Doe has a cholesterol (C27H46O)count of 178 mg/dL.You would like to calculate the number of cholesterol molecules that Jane Doe has in each deciliter of blood.Which of the following calculations is used to solve this problem?
A)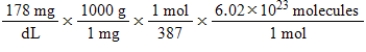
B)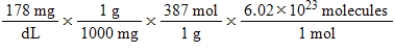
C)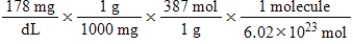
D)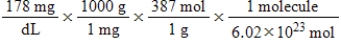
E)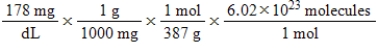
A)
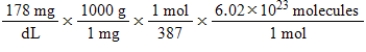
B)
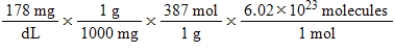
C)
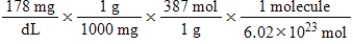
D)
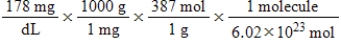
E)
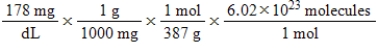

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
13
If Jane Doe has a blood carbon dioxide concentration of 0.022 mol/L, how many carbon dioxide molecules are in each liter of her blood?
A) 3.7 × 10-26
B) 0.022
C) 1.32 × 1022
D) 6.02 × 1023
E) 2.7 × 1025
A) 3.7 × 10-26
B) 0.022
C) 1.32 × 1022
D) 6.02 × 1023
E) 2.7 × 1025

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
14
Pentane (C5H12)reacts with oxygen gas (O2)to form carbon dioxide (CO2)and water (H2O)according to the following reaction.What is the coefficient for carbon dioxide in the balanced equation? C5H12 + ?O2 → ?CO2 + ?H2O
A) 2
B) 4
C) 5
D) 6
E) 8
A) 2
B) 4
C) 5
D) 6
E) 8

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
15
Under what circumstances is mass conserved?
A) Mass is conserved only in the laboratory.
B) Mass is conserved only theoretically.
C) Mass is conserved everywhere except the human body.
D) Mass is conserved in nature but not in the laboratory.
E) Mass is conserved during all chemical reactions.
A) Mass is conserved only in the laboratory.
B) Mass is conserved only theoretically.
C) Mass is conserved everywhere except the human body.
D) Mass is conserved in nature but not in the laboratory.
E) Mass is conserved during all chemical reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
16
What type of reaction occurs when hydrogen peroxide reacts to give water and oxygen? 2 H2O2 (l)→ 2 H2O (l)+ O2 (g)
A) combination
B) decomposition
C) single replacement
D) double replacement
E) oxidation-reduction
A) combination
B) decomposition
C) single replacement
D) double replacement
E) oxidation-reduction

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
17
Calculate the mass of one mole of fluoxetine, an antidepressant with the chemical formula C17H18F3NO.
A) 6.02 × 1023 g
B) 309 g
C) 162 g
D) 48.0 g
E) 40.0 g
A) 6.02 × 1023 g
B) 309 g
C) 162 g
D) 48.0 g
E) 40.0 g

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
18
If Jane Doe has a blood glucose (C6H12O6)concentration of 0.102 g/dL, how many glucose molecules does she have in each deciliter of blood?
A) 3.41 × 1026
B) 3.41 × 1023
C) 3.41 × 1020
D) 6.41 × 1022
E) 5.67 × 10-4
A) 3.41 × 1026
B) 3.41 × 1023
C) 3.41 × 1020
D) 6.41 × 1022
E) 5.67 × 10-4

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
19
Calculate the mass of one mole of dopamine, a neurotransmitter with the molecular formula C8H11NO2.
A) 22.0 g
B) 43.0 g
C) 82.0 g
D) 153 g
E) 164 g
A) 22.0 g
B) 43.0 g
C) 82.0 g
D) 153 g
E) 164 g

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
20
Which of these figures illustrate chemical reactions? 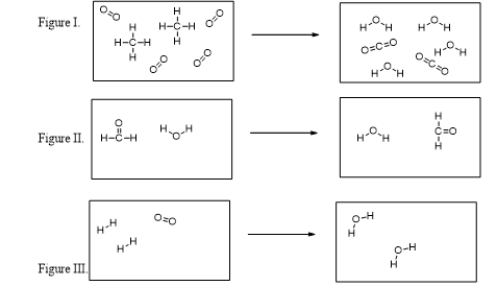
A) All of these are chemical reactions.
B) Only I and II are chemical reactions.
C) Only I and III are chemical reactions.
D) Only II and III are chemical reactions.
E) None of these figures represent chemical reactions.

A) All of these are chemical reactions.
B) Only I and II are chemical reactions.
C) Only I and III are chemical reactions.
D) Only II and III are chemical reactions.
E) None of these figures represent chemical reactions.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
21
The balanced chemical equation for the combustion of butane is given below and is the reaction happening when you use a butane lighter.How many grams of O2 are needed to completely react with 5.0 g of butane? 2 C4H10 + 13 O2 → 8 CO2 + 10 H2O
A) 0.42 g
B) 2.8 g
C) 6.0 g
D) 9.1 g
E) 18 g
A) 0.42 g
B) 2.8 g
C) 6.0 g
D) 9.1 g
E) 18 g

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following transformations is a chemical reaction?
A) water boiling
B) burning wood
C) condensation on the outside of a water glass
D) dry ice changing from a solid to a gas
E) evaporation of water from a glass
A) water boiling
B) burning wood
C) condensation on the outside of a water glass
D) dry ice changing from a solid to a gas
E) evaporation of water from a glass

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following statements describes the processes that can occur during oxidation? I.Loss of electrons
II)Gain of electrons
III)Loss of oxygens
IV)Loss of hydrogens
A) I only
B) II only
C) I and III
D) I and IV
E) II and IV
II)Gain of electrons
III)Loss of oxygens
IV)Loss of hydrogens
A) I only
B) II only
C) I and III
D) I and IV
E) II and IV

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
24
Jane Doe has a cholesterol (C27H46O)count of 178 mg/dL.You would like to calculate the number of cholesterol molecules that Jane Doe has in each deciliter of blood.Which of the following unit conversions are required to calculate the number of cholesterol molecules per deciliter?
i.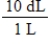
II.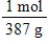
III.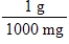
IV.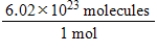
A) All of the conversions are needed.
B) I and IV
C) III and IV
D) I, II, and III
E) II, III, and IV
i.
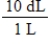
II.
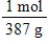
III.
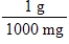
IV.
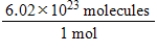
A) All of the conversions are needed.
B) I and IV
C) III and IV
D) I, II, and III
E) II, III, and IV

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
25
How is a nuclear reaction different from a chemical reaction?
A) They are identical.
B) Only in a chemical reaction can atoms change into different atoms.
C) Chemical reactions involve only the nucleus of the atom.
D) Only in a nuclear reaction can atoms change into different atoms.
E) Nuclear reactions release energy and chemical reactions absorb energy.
A) They are identical.
B) Only in a chemical reaction can atoms change into different atoms.
C) Chemical reactions involve only the nucleus of the atom.
D) Only in a nuclear reaction can atoms change into different atoms.
E) Nuclear reactions release energy and chemical reactions absorb energy.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the figures violates the conservation of mass? 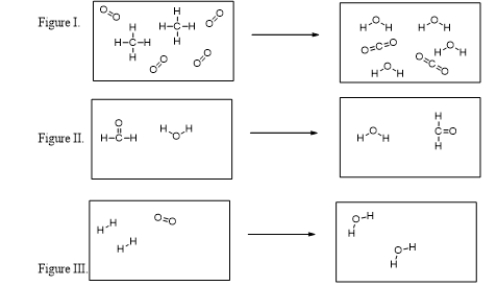
A) All of these figures violate the conservation of mass.
B) Figure I only
C) Figure II only
D) Figure III only
E) Figures II and III both violate the conservation of mass.

A) All of these figures violate the conservation of mass.
B) Figure I only
C) Figure II only
D) Figure III only
E) Figures II and III both violate the conservation of mass.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
27
The millions of chemical reactions that occur every second in a biological cell are ________________.
A) the cell's metabolic reactions
B) cell death
C) the organism's genetic blueprint
D) digestion
E) unrelated to the processes necessary for life
A) the cell's metabolic reactions
B) cell death
C) the organism's genetic blueprint
D) digestion
E) unrelated to the processes necessary for life

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
28
Your friend combines vinegar (which contains acetic acid, CH3COOH)and baking soda (NaHCO3)and ends up with a runny foam.Your friend is pretty sure that the reaction produces water and carbon dioxide because the reaction forms bubbles, but he is unsure of what else the reaction produces.Given the partial reaction below, what do you tell your friend? NaHCO3 + CH3COOH → H2O + CO2 + ?
A) This reaction also produces sodium acetate (CH3COONa).
B) This reaction also produces methane (CH4).
C) This reaction also produces O2.
D) This reaction only produces H2O and CO2.
E) This reaction probably produces something else, but it is not possible to determine the identity of the other product(s).
A) This reaction also produces sodium acetate (CH3COONa).
B) This reaction also produces methane (CH4).
C) This reaction also produces O2.
D) This reaction only produces H2O and CO2.
E) This reaction probably produces something else, but it is not possible to determine the identity of the other product(s).

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
29
How many molecules of glucose are in 1 mole of glucose?
A) 3
B) 24
C) 29
D) 180
E) 6.02 × 1023
A) 3
B) 24
C) 29
D) 180
E) 6.02 × 1023

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
30
The following reaction is an example of a(n)___________ reaction. CH4 + 2 O2 → CO2 + 2 H2O
I.oxidation-reduction
II.combination
III.combustion
A) I only
B) I and II only
C) III only
D) I and III only
E) I, II, and III
I.oxidation-reduction
II.combination
III.combustion
A) I only
B) I and II only
C) III only
D) I and III only
E) I, II, and III

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
31
What is the balanced chemical equation for the reaction illustrated in Figure I? 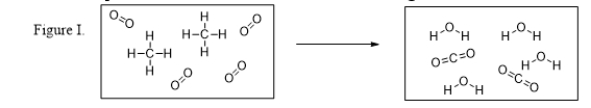
A) CH4 + 2 O2 → 2 H2O + CO2
B) CH4 + O2 → H2O + CO2
C) 2 CH4 + 4 O2 → 4 H2O + 2 CO2
D) 2 C + 8 H + 8 O → 2 C + 8 H + 8 O
E) C + 4 H + 4 O → C + 4 H + 4 O

A) CH4 + 2 O2 → 2 H2O + CO2
B) CH4 + O2 → H2O + CO2
C) 2 CH4 + 4 O2 → 4 H2O + 2 CO2
D) 2 C + 8 H + 8 O → 2 C + 8 H + 8 O
E) C + 4 H + 4 O → C + 4 H + 4 O

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
32
How many grams of iron are in 1.28 mol of iron?
A) 0.0230 g
B) 20.3 g
C) 33.3 g
D) 43.6 g
E) 71.4 g
A) 0.0230 g
B) 20.3 g
C) 33.3 g
D) 43.6 g
E) 71.4 g

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
33
In the body, glucose is broken down in the presence of oxygen into carbon dioxide and water.The balanced chemical equation for this reaction is shown below.According to this equation, how many molecules of carbon dioxide are produced when three molecules of glucose are metabolized in the presence of 18 moles of oxygen?
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
A) 0
B) 6
C) 12
D) 18
E) 24
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
A) 0
B) 6
C) 12
D) 18
E) 24

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
34
How many copper atoms are in 0.5 mol of copper?
A) 6.02 × 1012
B) 0.5
C) 3.01 × 1023
D) 32
E) 3
A) 6.02 × 1012
B) 0.5
C) 3.01 × 1023
D) 32
E) 3

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following characteristics is NOT shared by all combustion reactions?
A) They occur in a combustion engine.
B) They require oxygen.
C) They release water and carbon dioxide.
D) They give off heat.
E) They are a type of oxidation-reduction reaction.
A) They occur in a combustion engine.
B) They require oxygen.
C) They release water and carbon dioxide.
D) They give off heat.
E) They are a type of oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
36
How many atoms of magnesium are present in the reactants of this chemical equation? Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
37
What are the products of the reaction illustrated in Figure I? 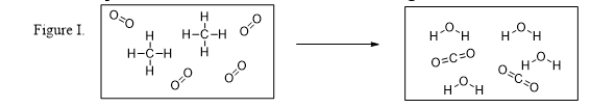
A) CH4 and O2
B) CO2 and H2O
C) CH4 only
D) O2 only
E) CO2 only

A) CH4 and O2
B) CO2 and H2O
C) CH4 only
D) O2 only
E) CO2 only

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
38
Which statement describes how formula mass and molecular mass differ?
A) A formula mass is measured in atomic mass units, whereas a molecular mass is measured in grams per mole.
B) A formula mass is measured in grams per mole, whereas a molecular mass is measured in atomic mass units.
C) A formula mass is the mass of one formula unit, whereas molecular mass is the mass of one molecule.
D) A formula mass is the mass of one molecule, whereas molecular mass is the mass of one formula unit.
E) There is no difference between these terms.
A) A formula mass is measured in atomic mass units, whereas a molecular mass is measured in grams per mole.
B) A formula mass is measured in grams per mole, whereas a molecular mass is measured in atomic mass units.
C) A formula mass is the mass of one formula unit, whereas molecular mass is the mass of one molecule.
D) A formula mass is the mass of one molecule, whereas molecular mass is the mass of one formula unit.
E) There is no difference between these terms.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
39
How many atoms of hydrogen are present in the products of this chemical equation? Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is NOT an example of a double displacement reaction?
A) BaSO4 + CuCl2 → BaCl2 + CuSO4
B) 2 KI (aq) + Pb(NO3)2 (aq) → 2 KNO3 (aq) + PbI2 (s)
C)
D) CH4 + 2O2 → CO2 + 2H2O
E) NaOH + HCl → H2O + NaCl
A) BaSO4 + CuCl2 → BaCl2 + CuSO4
B) 2 KI (aq) + Pb(NO3)2 (aq) → 2 KNO3 (aq) + PbI2 (s)
C)

D) CH4 + 2O2 → CO2 + 2H2O
E) NaOH + HCl → H2O + NaCl

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following correctly determines the formula mass of sodium sulfate (Na2SO4)?
A) 7 × (23 amu + 32 amu + 16 amu)
B) 2 × 23 amu + 4 × (32 amu + 16 amu)
C) 2 × 23 amu + 32 amu + 4 × 16 amu
D) 2 × 11 amu + 16 amu + 4 × 8 amu
E) 2 × 11 amu + 4 × (16 amu + 8 amu)
A) 7 × (23 amu + 32 amu + 16 amu)
B) 2 × 23 amu + 4 × (32 amu + 16 amu)
C) 2 × 23 amu + 32 amu + 4 × 16 amu
D) 2 × 11 amu + 16 amu + 4 × 8 amu
E) 2 × 11 amu + 4 × (16 amu + 8 amu)

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
42
How many moles of the antibiotic tetracycline (C22H24N2O8)are in a 50-mg dose?
A) 8880 mol
B) 22.2 mol
C) 8.88 mol
D) 0.113 mol
E) 1.13 × 10-4 mol
A) 8880 mol
B) 22.2 mol
C) 8.88 mol
D) 0.113 mol
E) 1.13 × 10-4 mol

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
43
An oxidation-reduction reaction is the transfer of ________.
A) sodium ions
B) phosphate ions
C) protons
D) electrons
E) oxygen atoms
A) sodium ions
B) phosphate ions
C) protons
D) electrons
E) oxygen atoms

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
44
Coefficients are important components of chemical equations.What is the significance of coefficients in a chemical equation? I.Coefficients indicate the ratio of masses of molecules.
II)Coefficients indicate the ratio of numbers of atoms and molecules.
III)Coefficients indicate the ratio of numbers of moles of molecules and atoms.
A) I only
B) II only
C) III only
D) I and II
E) II and III
II)Coefficients indicate the ratio of numbers of atoms and molecules.
III)Coefficients indicate the ratio of numbers of moles of molecules and atoms.
A) I only
B) II only
C) III only
D) I and II
E) II and III

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
45
The ionic compound CaCO3 has a formula mass of:
A) 50 g/mol.
B) 50 amu.
C) 100 g/mol.
D) 100 amu.
E) 68.1 amu.
A) 50 g/mol.
B) 50 amu.
C) 100 g/mol.
D) 100 amu.
E) 68.1 amu.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
46
In which figure are new O-H bonds being formed? 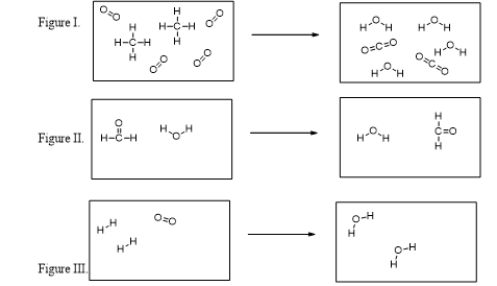
A) Figure I
B) Figure II
C) Figure III
D) Figures I and II
E) Figures I and III

A) Figure I
B) Figure II
C) Figure III
D) Figures I and II
E) Figures I and III

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
47
The following reaction is an example of a ___________ reaction. 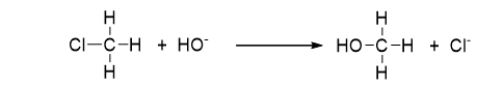
A) combination
B) decomposition
C) single replacement
D) double replacement
E) oxidation-reduction
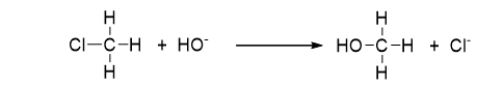
A) combination
B) decomposition
C) single replacement
D) double replacement
E) oxidation-reduction

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
48
In an oxidation-reduction reaction, the species that loses electrons is _______.
A) oxygen
B) oxidized
C) hydrogen
D) reduced
E) carbon
A) oxygen
B) oxidized
C) hydrogen
D) reduced
E) carbon

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
49
Acetylene (C2H2)is a small organic molecule used in the industrial preparation of many other molecules and also in welding.Acetylene is made from calcium carbide (CaC2)and water and produces acetylene and calcium hydroxide.Which of the following choices is the correct balanced chemical equation for the preparation of acetylene?
A) CaC2 + H2O → CaO + C2H2
B) CaC2 + H2O → Ca(OH)2 + C2H2
C) CaC2 + 2 H2O → Ca(OH)2 + C2H2
D) CaC2 + 2 H2O → CaO + C2H2 + H2O
E) 2 CaC2 + H2O → Ca(OH)2 + 2 C2H2
A) CaC2 + H2O → CaO + C2H2
B) CaC2 + H2O → Ca(OH)2 + C2H2
C) CaC2 + 2 H2O → Ca(OH)2 + C2H2
D) CaC2 + 2 H2O → CaO + C2H2 + H2O
E) 2 CaC2 + H2O → Ca(OH)2 + 2 C2H2

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is NOT one of the basic types of reactions?
A) ionic
B) combination
C) decomposition
D) single replacement
E) double replacement
A) ionic
B) combination
C) decomposition
D) single replacement
E) double replacement

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
51
Which statement BEST describes the following reaction? Fe + Cu2+ → Fe2+ + Cu
A) In this reaction, both iron and copper are reduced.
B) In this reaction, both iron and copper are oxidized.
C) In this reaction, iron is reduced, and copper is oxidized.
D) In this reaction, iron is oxidized, and copper is reduced.
E) This is not an oxidation-reduction reaction.
A) In this reaction, both iron and copper are reduced.
B) In this reaction, both iron and copper are oxidized.
C) In this reaction, iron is reduced, and copper is oxidized.
D) In this reaction, iron is oxidized, and copper is reduced.
E) This is not an oxidation-reduction reaction.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following chemical equations is balanced?
A) C3H8 + O2 → CO2 + H2O
B) C3H8 + O2 → 3 CO2 + 4 H2O
C) C3H8 + 10 O2 → 3 CO2 + 4 H2O
D) C3H8 + 5 O2 → 3 CO2 + 4 H2O
E) None of these are balanced.
A) C3H8 + O2 → CO2 + H2O
B) C3H8 + O2 → 3 CO2 + 4 H2O
C) C3H8 + 10 O2 → 3 CO2 + 4 H2O
D) C3H8 + 5 O2 → 3 CO2 + 4 H2O
E) None of these are balanced.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
53
Is this chemical equation balanced? Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O
A) No.The number of magnesiums in the reactants is different from the number in the products.
B) No.The number of hydrogens in the reactants is different from the number in the products.
C) No.The number of oxygens in the reactants is different from the number in the products.
D) No.The number of chlorines in the reactants is different from the number in the products.
E) Yes.The same number of each type of atom is present in the reactants and products.
A) No.The number of magnesiums in the reactants is different from the number in the products.
B) No.The number of hydrogens in the reactants is different from the number in the products.
C) No.The number of oxygens in the reactants is different from the number in the products.
D) No.The number of chlorines in the reactants is different from the number in the products.
E) Yes.The same number of each type of atom is present in the reactants and products.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
54
In an oxidation-reduction reaction, the species that gains electrons is _______.
A) oxygen
B) oxidized
C) hydrogen
D) reduced
E) carbon
A) oxygen
B) oxidized
C) hydrogen
D) reduced
E) carbon

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
55
Jane Doe has her cholesterol measured and the resulting lab report gives a value of 0.548 × 10-3 mol/dL.You know that the normal range of cholesterol (C27H46O)is 0.100-0.240 g/dL.Is Jane Doe's cholesterol within the normal range?
A) No, it is above 0.240 g/dL.
B) No, it is below 0.100 g/dL.
C) Yes, it is between 0.100 g/dL and 0.240 g/dL.
D) Yes, it is above 0.240 g/dL.
E) Yes, it is below 0.100 g/dL.
A) No, it is above 0.240 g/dL.
B) No, it is below 0.100 g/dL.
C) Yes, it is between 0.100 g/dL and 0.240 g/dL.
D) Yes, it is above 0.240 g/dL.
E) Yes, it is below 0.100 g/dL.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
56
The balanced chemical equation for the combustion of propane (C3H8)is given below.How many grams of carbon dioxide (CO2)are released when 35 g of propane are burned in the presence of excess oxygen? C3H8 + 5 O2 → 3 CO2 + 4 H2O
A) 0.80 g
B) 12 g
C) 35 g
D) 105 g
E) 1,500 g
A) 0.80 g
B) 12 g
C) 35 g
D) 105 g
E) 1,500 g

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
57
Which statement BEST describes the changes that occur over the course of the following oxidation-reduction reaction? Fe + Cu2+ → Fe2+ + Cu
A) Two electrons are lost.
B) Iron changes into copper.
C) Iron transfers two electrons to copper.
D) Copper transfers two electrons to iron.
E) Two electrons are gained.
A) Two electrons are lost.
B) Iron changes into copper.
C) Iron transfers two electrons to copper.
D) Copper transfers two electrons to iron.
E) Two electrons are gained.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
58
How many moles is 9.1 × 1024 ions of Na+?
A) 5.5 × 1048 mol
B) 0.17 mol
C) 1 mol
D) 15 mol
E) 0.67 mol
A) 5.5 × 1048 mol
B) 0.17 mol
C) 1 mol
D) 15 mol
E) 0.67 mol

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
59
A chemical reaction involves many changes but not everything changes during a reaction.Which of the following is NOT a change that occurs during a chemical reaction?
A) Atoms change in number or identity.
B) Bonds break.
C) Heat is released and/or absorbed.
D) New bonds form.
E) New molecules are formed.
A) Atoms change in number or identity.
B) Bonds break.
C) Heat is released and/or absorbed.
D) New bonds form.
E) New molecules are formed.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
60
Cellular respiration is an example of a(n)___________ reaction.
A) combination
B) decomposition
C) single replacement
D) double replacement
E) oxidation-reduction
A) combination
B) decomposition
C) single replacement
D) double replacement
E) oxidation-reduction

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following is a consideration in the design of air bags?
A) The chemical reaction must be very fast.
B) The reactants must be stable compounds.
C) The air bag must be able to fit into a small space when not inflated.
D) The chemical reaction must produce a gas.
E) All of the above are considerations.
A) The chemical reaction must be very fast.
B) The reactants must be stable compounds.
C) The air bag must be able to fit into a small space when not inflated.
D) The chemical reaction must produce a gas.
E) All of the above are considerations.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
62
How many moles of carbon are in 27.1 g of carbon?
A) 2.26 mol
B) 325 mol
C) 0.443 mol
D) 5.52 mol
E) 163 mol
A) 2.26 mol
B) 325 mol
C) 0.443 mol
D) 5.52 mol
E) 163 mol

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
63
Which statement BEST describes why we use moles when measuring quantities of atoms and molecules?
A) Moles are used because they are units of mass.
B) Moles are used because a mole is the same as the atomic mass of an element.
C) Moles are used because atoms and molecules are too small to measure singly.
D) Moles are used because atoms and molecules exist in units of moles.
E) Moles are not involved in measuring quantities of atoms and molecules.
A) Moles are used because they are units of mass.
B) Moles are used because a mole is the same as the atomic mass of an element.
C) Moles are used because atoms and molecules are too small to measure singly.
D) Moles are used because atoms and molecules exist in units of moles.
E) Moles are not involved in measuring quantities of atoms and molecules.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
64
Which statement describes how molecular mass and molar mass differ?
A) Molecular mass is the mass of one molecule, whereas molar mass is the mass of one mole.
B) Molar mass is measured in atomic mass units, whereas molecular mass is measured in grams per mole.
C) A molar mass is the mass of one formula unit, whereas molecular mass is the mass of one molecule.
D) A molar mass is the mass of one molecule, whereas molecular mass is the mass of one formula unit.
E) There is no difference between these terms.
A) Molecular mass is the mass of one molecule, whereas molar mass is the mass of one mole.
B) Molar mass is measured in atomic mass units, whereas molecular mass is measured in grams per mole.
C) A molar mass is the mass of one formula unit, whereas molecular mass is the mass of one molecule.
D) A molar mass is the mass of one molecule, whereas molecular mass is the mass of one formula unit.
E) There is no difference between these terms.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
65
The reaction that occurs in a hydrogen fuel cell is shown below.According to the chemical reaction, the product(s)of the reaction is/are __________, which is one of the benefits of the fuel cell. 2 H2 (g)+ O2 (g)→ 2 H2O (g)
A) carbon dioxide
B) a liquid
C) hydrogen and oxygen
D) hydrogen
E) water
A) carbon dioxide
B) a liquid
C) hydrogen and oxygen
D) hydrogen
E) water

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following equations is used to convert 91.0 milligrams of urea (CH4N2O)to moles of urea?
A)
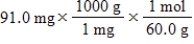
B)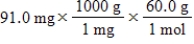
C)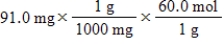
D)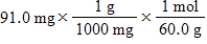
E)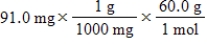
A)

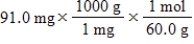
B)
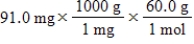
C)
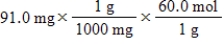
D)
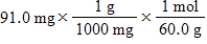
E)
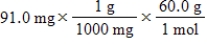

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
67
What is the mass of one mole of the hydroxide ion (OH-)?
A) 8 g
B) 15 g
C) 17 g
D) 7 g
E) 5 g
A) 8 g
B) 15 g
C) 17 g
D) 7 g
E) 5 g

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following is NOT indicated by a chemical equation?
A) the formulas of the reactants and products of a chemical reaction
B) the ratio of reactants and products in a chemical reaction
C) how fast a chemical reaction proceeds
D) the quantitative relationship between reactants and products in a chemical reaction
E) the state of matter of reactants and products
A) the formulas of the reactants and products of a chemical reaction
B) the ratio of reactants and products in a chemical reaction
C) how fast a chemical reaction proceeds
D) the quantitative relationship between reactants and products in a chemical reaction
E) the state of matter of reactants and products

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
69
The balanced equation for the combustion of propane is given below.What do the numbers in front of the molecules (the coefficients)mean? C3H8 + 5 O2 → 3 CO2 + 4 H2O
A) Three molecules of CO2 are produced when one molecule of C3H8 reacts with one molecule of O2.
B) Six molecules of CO2 are produced when two molecules of C3H8 react with two molecules of O2.
C) Nine moles of CO2 are produced when three moles of C3H8 react with three moles of O2.
D) Four moles of H2O are produced when one mole of C3H8 reacts with one mole of O2.
E) All of the above representations are correct.
A) Three molecules of CO2 are produced when one molecule of C3H8 reacts with one molecule of O2.
B) Six molecules of CO2 are produced when two molecules of C3H8 react with two molecules of O2.
C) Nine moles of CO2 are produced when three moles of C3H8 react with three moles of O2.
D) Four moles of H2O are produced when one mole of C3H8 reacts with one mole of O2.
E) All of the above representations are correct.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
70
How many carbon atoms are in 1 mole of carbon?
A) 1
B) 12
C) 12.011
D) 6
E) 6.02 × 1023
A) 1
B) 12
C) 12.011
D) 6
E) 6.02 × 1023

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
71
Is methane (CH4)undergoing oxidation or reduction during this reaction, and how can you tell? CH4 + 2 O2 → CO2 + 2 H2O
A) Methane is oxidized because there is an increase in oxygen and a decrease in hydrogen.
B) Methane is reduced because there is an increase in oxygen and a decrease in hydrogen.
C) Methane is oxidized because it becomes a gas.
D) Methane is reduced because water is formed.
E) Methane is reduced because carbon becomes a gas.
A) Methane is oxidized because there is an increase in oxygen and a decrease in hydrogen.
B) Methane is reduced because there is an increase in oxygen and a decrease in hydrogen.
C) Methane is oxidized because it becomes a gas.
D) Methane is reduced because water is formed.
E) Methane is reduced because carbon becomes a gas.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
72
What type of reaction is the addition of hydrochloric acid (HCl)to propene? 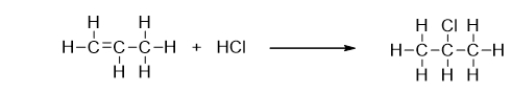
A) combination
B) decomposition
C) single replacement
D) double replacement
E) oxidation-reduction

A) combination
B) decomposition
C) single replacement
D) double replacement
E) oxidation-reduction

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
73
The following reaction is the formation of a peptide from two amino acids, the sort of reaction that occurs in the body as part of the synthesis of proteins.This reaction is missing a component.Which of the following is missing? 2 NH2CH2COOH → NH2CH2CONHCH2COOH
A) Nothing is missing.
B) H2O is missing from the reactant side of the equation.
C) H2O is missing from the product side of the equation.
D) O2 is missing from the reactant side of the equation.
E) O2 is missing from the product side of the equation.
A) Nothing is missing.
B) H2O is missing from the reactant side of the equation.
C) H2O is missing from the product side of the equation.
D) O2 is missing from the reactant side of the equation.
E) O2 is missing from the product side of the equation.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
74
Does one mole of the antibiotic penicillin G (C16H18N2O4S)weigh more or less than one mole of the antibiotic streptomycin (C21H39N7O12)?
A) They weigh the same because a mole always has the same mass.
B) They weigh the same because a mole always has the same number of particles in it.
C) Streptomycin weighs more because its individual molecules are larger.
D) Penicillin weighs more because its individual molecules are larger.
E) It is not possible to determine which one would weigh more because mole size varies.
A) They weigh the same because a mole always has the same mass.
B) They weigh the same because a mole always has the same number of particles in it.
C) Streptomycin weighs more because its individual molecules are larger.
D) Penicillin weighs more because its individual molecules are larger.
E) It is not possible to determine which one would weigh more because mole size varies.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
75
What does it mean for a chemical equation to be balanced?
A) A balanced chemical equation describes a chemical reaction found in nature.
B) A balanced chemical equation describes a chemical reaction that produces only some product, so there is an equal amount of reactant and product.
C) A balanced chemical equation contains an equal number of each type of atoms on both sides of the equation.
D) A balanced chemical equation can run either forward or backward.
E) A balanced chemical equation represents a chemical reaction that is part of metabolism.
A) A balanced chemical equation describes a chemical reaction found in nature.
B) A balanced chemical equation describes a chemical reaction that produces only some product, so there is an equal amount of reactant and product.
C) A balanced chemical equation contains an equal number of each type of atoms on both sides of the equation.
D) A balanced chemical equation can run either forward or backward.
E) A balanced chemical equation represents a chemical reaction that is part of metabolism.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
76
Jane Doe has a cholesterol (C27H46O)count of 178 mg/dL.How many cholesterol molecules does Jane Doe have in each deciliter of blood?
A) 7.64 × 10-22
B) 1.14 × 10-22
C) 2.77 × 1020
D) 5.54 × 1025
E) 2.77 × 1026
A) 7.64 × 10-22
B) 1.14 × 10-22
C) 2.77 × 1020
D) 5.54 × 1025
E) 2.77 × 1026

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
77
A solution of glucose has 10.00 g glucose (molecular weight = 180.g/mol)in 1.00 L of water.How many molecules of glucose are in 1.00 L of the solution?
A) 0.05 molecules
B) 180 molecules
C) 3.34 ×1022 molecules
D) 6.02 × 1023 molecules
E) 6.02 × 1024 molecules
A) 0.05 molecules
B) 180 molecules
C) 3.34 ×1022 molecules
D) 6.02 × 1023 molecules
E) 6.02 × 1024 molecules

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
78
Hydrogen peroxide loses its potency when it is stored for extended periods of time because it spontaneously breaks down.The following figure illustrates this breakdown, but it is missing a product molecule.Which of the following symbols represents the missing product in this reaction? 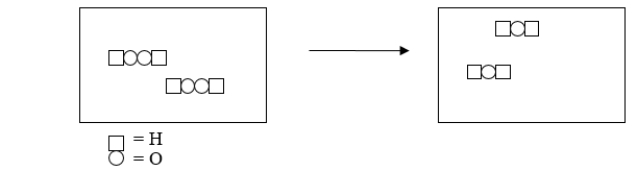
A)
B)
C)
D)
E)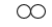

A)
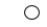
B)

C)

D)

E)
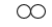

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
79
The following chemical equation, the decomposition of hydrogen peroxide to water, is not balanced.What must be done to the equation to make it balanced? 2 H2O2(l)→ 2 H2O(l)
A) Change the coefficients from 2 to 1.
B) Add an atom of oxygen to the products.
C) Add a molecule of O2 to the products.
D) Change the state of matter in the products.
E) Change the subscripts of H2O to H2O2.
A) Change the coefficients from 2 to 1.
B) Add an atom of oxygen to the products.
C) Add a molecule of O2 to the products.
D) Change the state of matter in the products.
E) Change the subscripts of H2O to H2O2.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck
80
What is the meaning of the arrow in the following chemical equation? 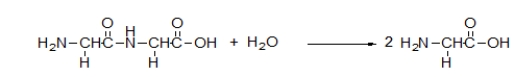
A) The reaction mixture increases in temperature.
B) A product is substituted for a reactant.
C) The reactants collide.
D) The reactants are transformed into the products.
E) The reaction is heated.
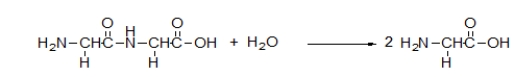
A) The reaction mixture increases in temperature.
B) A product is substituted for a reactant.
C) The reactants collide.
D) The reactants are transformed into the products.
E) The reaction is heated.

Unlock Deck
Unlock for access to all 88 flashcards in this deck.
Unlock Deck
k this deck


