Exam 5: Chemical Quantities and Introduction to Reactions
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Calculate the mass of one mole of fluoxetine, an antidepressant with the chemical formula C17H18F3NO.
Free
(Multiple Choice)
4.9/5  (25)
(25)
Correct Answer:
B
How many moles is 9.1 × 1024 ions of Na+?
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
D
The balanced equation for the reaction of nitrogen and hydrogen to form ammonia is given below.What do the numbers in front of the molecules (the coefficients)mean? N2 + 3 H2 → 2 NH3
Free
(Multiple Choice)
5.0/5  (26)
(26)
Correct Answer:
A
What type of reaction occurs when hydrogen peroxide reacts to give water and oxygen? 2 H2O2 (l)→ 2 H2O (l)+ O2 (g)
(Multiple Choice)
4.8/5  (38)
(38)
Pentane (C5H12)reacts with oxygen gas (O2)to form carbon dioxide (CO2)and water (H2O)according to the following reaction.What is the coefficient for water in the balanced equation? C5H12 + ?O2 → ?CO2 + ?H2O
(Multiple Choice)
4.9/5  (30)
(30)
The industrial process for making ammonia from nitrogen and hydrogen gases is called the Haber process and is used in the manufacture of fertilizers and other nitrogen-containing compounds.The chemical equation for this process is shown below.According to this reaction, how many moles of ammonia would you produce if you reacted one mole of N2 with three moles of H2? N2 + 3 H2 → 2 NH3
(Multiple Choice)
4.9/5  (37)
(37)
How many moles of the antibiotic tetracycline (C22H24N2O8)are in a 50-mg dose?
(Multiple Choice)
4.9/5  (28)
(28)
Pentane (C5H12)reacts with oxygen gas (O2)to form carbon dioxide (CO2)and water (H2O)according to the following reaction.What is the coefficient for carbon dioxide in the balanced equation? C5H12 + ?O2 → ?CO2 + ?H2O
(Multiple Choice)
4.7/5  (31)
(31)
If Jane Doe has a blood glucose (C6H12O6)concentration of 0.102 g/dL, how many glucose molecules does she have in each deciliter of blood?
(Multiple Choice)
4.7/5  (33)
(33)
The reaction that occurs in a hydrogen fuel cell is shown below.According to the chemical reaction, the product(s)of the reaction is/are __________, which is one of the benefits of the fuel cell. 2 H2 (g)+ O2 (g)→ 2 H2O (g)
(Multiple Choice)
4.9/5  (34)
(34)
In an oxidation-reduction reaction, the species that loses electrons is _______.
(Multiple Choice)
4.8/5  (30)
(30)
An oxidation-reduction reaction is the transfer of ________.
(Multiple Choice)
4.7/5  (36)
(36)
Jane Doe has her cholesterol measured and the resulting lab report gives a value of 0.548 × 10-3 mol/dL.You know that the normal range of cholesterol (C27H46O)is 0.100-0.240 g/dL.Is Jane Doe's cholesterol within the normal range?
(Multiple Choice)
4.9/5  (28)
(28)
Your friend combines vinegar (which contains acetic acid, CH3COOH)and baking soda (NaHCO3)and ends up with a runny foam.Your friend is pretty sure that the reaction produces water and carbon dioxide because the reaction forms bubbles, but he is unsure of what else the reaction produces.Given the partial reaction below, what do you tell your friend? NaHCO3 + CH3COOH → H2O + CO2 + ?
(Multiple Choice)
4.9/5  (28)
(28)
How many atoms of hydrogen are present in the products of this chemical equation? Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O
(Multiple Choice)
4.8/5  (31)
(31)
Hydrogen peroxide loses its potency when it is stored for extended periods of time because it spontaneously breaks down.The following figure illustrates this breakdown, but it is missing a product molecule.Which of the following symbols represents the missing product in this reaction? 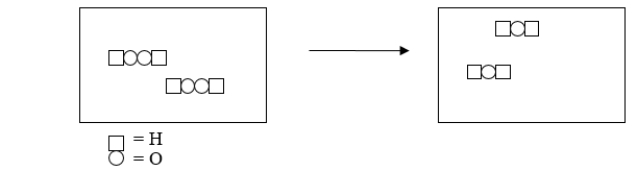
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)