Deck 3: Ionic and Covalent Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/95
Play
Full screen (f)
Deck 3: Ionic and Covalent Compounds
1
Which element forms a cation?
A) carbon
B) phosphorus
C) krypton
D) nickel
E) iodine
A) carbon
B) phosphorus
C) krypton
D) nickel
E) iodine
nickel
2
People on a low salt diet use a potassium chloride substitute for sodium chloride.Which of the following is a valid ionic formula for potassium chloride?
A) NaCl
B) NaCl2
C) KCl2
D) KCl
E) K2Cl
A) NaCl
B) NaCl2
C) KCl2
D) KCl
E) K2Cl
KCl
3
What is the charge on iron in the ionic compound FeCl3?
A) +3
B) +1
C) 0
D) -1
E) -3
A) +3
B) +1
C) 0
D) -1
E) -3
+3
4
What is the total number of valence electrons in the chloroethylene molecule (C2H3Cl)?
A) 6
B) 18
C) 21
D) 32
E) 62
A) 6
B) 18
C) 21
D) 32
E) 62

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
5
What is the meaning of the symbol below? [PO4]3-
A) The phosphorous has three more electrons than protons.
B) Three oxygens each have one more electron than protons.
C) Each of the four oxygens has three more electrons than protons.
D) The ion has three more electrons than protons.
E) The 3- means that the molecule has lost three electrons.
A) The phosphorous has three more electrons than protons.
B) Three oxygens each have one more electron than protons.
C) Each of the four oxygens has three more electrons than protons.
D) The ion has three more electrons than protons.
E) The 3- means that the molecule has lost three electrons.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
6
Calcium carbonate has the formula CaCO3.What is the charge on the polyatomic carbonate ion?
A) +2
B) +1
C) 0
D) -1
E) -2
A) +2
B) +1
C) 0
D) -1
E) -2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
7
Select the statement that BEST differentiates between bonding and nonbonding electrons.
A) There are always more bonding electrons than lone pairs in a molecule.
B) Nonbonding electrons are less chemically important than bonding electrons.
C) Nonbonding electrons do not count toward an atom's octet of electrons.
D) Bonding electrons are shared and nonbonding electrons are not shared.
E) The more nonbonding electrons that an atom has, the more unstable the atom is.
A) There are always more bonding electrons than lone pairs in a molecule.
B) Nonbonding electrons are less chemically important than bonding electrons.
C) Nonbonding electrons do not count toward an atom's octet of electrons.
D) Bonding electrons are shared and nonbonding electrons are not shared.
E) The more nonbonding electrons that an atom has, the more unstable the atom is.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following statements about ionic compounds is NOT true?
A) Ions that make up ionic compounds have the same properties as the elements from which they are formed.
B) Many ionic compounds are soluble in water.
C) Ionic compounds have high melting points.
D) Ions dissolved in water conduct electricity.
E) Ionic compounds are composed of cations and anions.
A) Ions that make up ionic compounds have the same properties as the elements from which they are formed.
B) Many ionic compounds are soluble in water.
C) Ionic compounds have high melting points.
D) Ions dissolved in water conduct electricity.
E) Ionic compounds are composed of cations and anions.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the structures are covalent compounds? 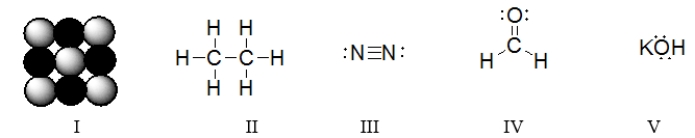
A) I
B) II, III, and IV
C) II and IV
D) V
E) All of the choices are covalent molecules.
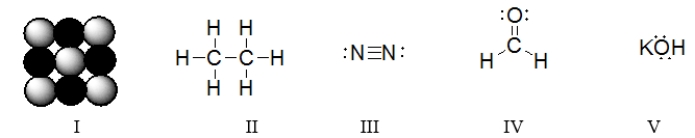
A) I
B) II, III, and IV
C) II and IV
D) V
E) All of the choices are covalent molecules.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
10
Oxygen is a _______ and therefore _______ when it forms an ion.
A) metal; loses electrons
B) metal; gains electrons
C) nonmetal; loses electrons
D) nonmetal; gains electrons
E) metalloid; loses electrons
A) metal; loses electrons
B) metal; gains electrons
C) nonmetal; loses electrons
D) nonmetal; gains electrons
E) metalloid; loses electrons

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
11
How many bonds is chlorine MOST likely to form?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is NOT composed of polyatomic ions?
A) table salt
B) bleach
C) some preservatives
D) tooth enamel
E) some bactericides
A) table salt
B) bleach
C) some preservatives
D) tooth enamel
E) some bactericides

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
13
Lewis electron dot structures represent the
A) number of total electrons in a molecule.
B) total number of electrons in the atom.
C) number of protons in the atom.
D) number of valence electrons.
E) number of nonbonding electrons.
A) number of total electrons in a molecule.
B) total number of electrons in the atom.
C) number of protons in the atom.
D) number of valence electrons.
E) number of nonbonding electrons.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
14
How many multiple bonds are in the chloroethylene molecule (C2H3Cl)?
A) zero multiple bonds
B) 1 double bond
C) 1 triple bond
D) 2 double bonds
E) 1 double and 1 triple bond
A) zero multiple bonds
B) 1 double bond
C) 1 triple bond
D) 2 double bonds
E) 1 double and 1 triple bond

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
15
What is the ionic formula for the ionic compound composed of calcium and chloride ions?
A) Ca2Cl
B) CaCl
C) Ca2Cl2
D) Ca2Cl3
E) CaCl2
A) Ca2Cl
B) CaCl
C) Ca2Cl2
D) Ca2Cl3
E) CaCl2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
16
"Laughing gas" has the formula N2O.Which of the following is the best name for this compound?
A) nitrogen oxygen
B) nitrogen oxide
C) nitrogen dioxide
D) dinitrogen monoxide
E) nitrogen(II)oxide
A) nitrogen oxygen
B) nitrogen oxide
C) nitrogen dioxide
D) dinitrogen monoxide
E) nitrogen(II)oxide

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is the formula for an ionic compound called iron(III)oxide?
A) Fe3O2
B) Fe2O3
C) FeO
D) Fe3O
E) FeO3
A) Fe3O2
B) Fe2O3
C) FeO
D) Fe3O
E) FeO3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
18
Which statement BEST describes the meaning of an expanded octet?
A) "Expanded octets" are molecules in which an element expands its valence shell to gain an octet of electrons.
B) "Expanded octets" is a term that describes atoms that have more than eight electrons in their valence shells.
C) "Expanded octets" refers to atoms that, when bonded, must be larger than normal.
D) "Expanded octets" is used to describe elements that do not normally form an octet when bonded and would therefore have to expand to form the octet.
E) "Expanded octets" is used to describe how atoms accept electrons to attain a full valence shell.
A) "Expanded octets" are molecules in which an element expands its valence shell to gain an octet of electrons.
B) "Expanded octets" is a term that describes atoms that have more than eight electrons in their valence shells.
C) "Expanded octets" refers to atoms that, when bonded, must be larger than normal.
D) "Expanded octets" is used to describe elements that do not normally form an octet when bonded and would therefore have to expand to form the octet.
E) "Expanded octets" is used to describe how atoms accept electrons to attain a full valence shell.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements about caffeine is TRUE?
A) Caffeine is a compound.
B) It is consumed by the majority of North Americans.
C) Caffeine binds to the adenosine receptor.
D) Caffeine activates the pleasure centers of the brain.
E) All of the above statements about caffeine are true.
A) Caffeine is a compound.
B) It is consumed by the majority of North Americans.
C) Caffeine binds to the adenosine receptor.
D) Caffeine activates the pleasure centers of the brain.
E) All of the above statements about caffeine are true.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following Lewis dot structures best represents the chlorine atom? 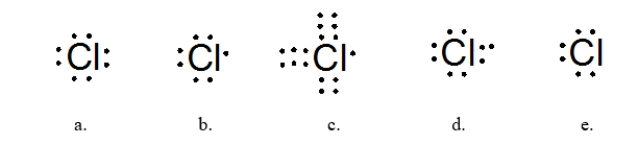
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is NOT a valid ionic formula?
A) NaF
B) MgO
C) KCl2
D) CaF2
E) Na2O
A) NaF
B) MgO
C) KCl2
D) CaF2
E) Na2O

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
22
Which group of elements forms anions?
A) noble gases
B) transition metals
C) alkaline metals
D) alkali earth metals
E) halogens
A) noble gases
B) transition metals
C) alkaline metals
D) alkali earth metals
E) halogens

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
23
Which is the BEST Lewis structure for a molecule with the formula HCN? 
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e
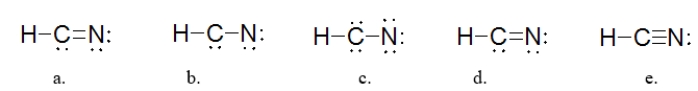
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
24
Elements in group 1A of the periodic table are likely to form ions with the charge
A) -3.
B) -2.
C) -1.
D) +1.
E) +2.
A) -3.
B) -2.
C) -1.
D) +1.
E) +2.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
25
In a covalent molecule
A) nonmetals share electrons.
B) nonmetals donate electrons to metals.
C) metals donate electrons to nonmetals.
D) metals share electrons.
E) cations and anions are attracted to one another.
A) nonmetals share electrons.
B) nonmetals donate electrons to metals.
C) metals donate electrons to nonmetals.
D) metals share electrons.
E) cations and anions are attracted to one another.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
26
How many nonbonding electrons are in the chloroethylene molecule (C2H3Cl)?
A) zero
B) 6
C) 8
D) 10
E) 18
A) zero
B) 6
C) 8
D) 10
E) 18

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following is a diatomic element?
A) N2
B) NaCl
C) H2O
D) CO
E) Li2O
A) N2
B) NaCl
C) H2O
D) CO
E) Li2O

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
28
Carbon tetrafluoride is a refrigerant and potent greenhouse gas.Which of the following is the molecular formula for this compound?
A) CF
B) C(IV)F
C) C4F4
D) C4F
E) CF4
A) CF
B) C(IV)F
C) C4F4
D) C4F
E) CF4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following ions is a polyatomic ion?
A) NH4+
B) Mg2+
C) O2-
D) O2
E) C2H6
A) NH4+
B) Mg2+
C) O2-
D) O2
E) C2H6

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
30
What is the BEST name for the compound SF6, a potent greenhouse gas?
A) sulfur fluorine
B) sulfur fluoride
C) sulfur hexafluoride
D) sulfur pentafluoride
E) monosulfur fluoride
A) sulfur fluorine
B) sulfur fluoride
C) sulfur hexafluoride
D) sulfur pentafluoride
E) monosulfur fluoride

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
31
What is the name of the ionic compound that is composed of calcium and chlorine?
A) calcium dichloride
B) dicalcium trichloride
C) dicalcium chloride
D) calcium chloride
E) dicalcium dichloride
A) calcium dichloride
B) dicalcium trichloride
C) dicalcium chloride
D) calcium chloride
E) dicalcium dichloride

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following items is NOT composed of an ionic compound?
A) caffeine
B) some sunscreens
C) calamine lotion
D) most toothpaste
E) table salt
A) caffeine
B) some sunscreens
C) calamine lotion
D) most toothpaste
E) table salt

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is NOT a monoatomic electrolyte in the body?
A) sodium ion
B) potassium ion
C) oxygen
D) calcium ion
E) chloride ion
A) sodium ion
B) potassium ion
C) oxygen
D) calcium ion
E) chloride ion

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
34
An atom of magnesium is shown below.The charge on this ion is _______ making the ion a _______. 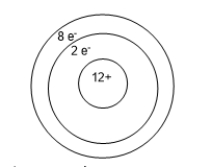
A) -10; anion
B) -2; anion
C) -2; cation
D) + 2; anion
E) + 2; cation

A) -10; anion
B) -2; anion
C) -2; cation
D) + 2; anion
E) + 2; cation

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
35
Elements in group 17 (7A)of the periodic table are likely to form ions with the charge
A) -3.
B) -2.
C) -1.
D) +1.
E) +2.
A) -3.
B) -2.
C) -1.
D) +1.
E) +2.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
36
An important part of writing a formula unit for an ionic compound is to make sure that
A) the charges of the ions add up to zero.
B) there is never more than one type of cation and one type of anion in the formula.
C) the ionic compound actually exists in nature.
D) the anion is written first, followed by the cation.
E) the ratio of cation to anion is 1:1.
A) the charges of the ions add up to zero.
B) there is never more than one type of cation and one type of anion in the formula.
C) the ionic compound actually exists in nature.
D) the anion is written first, followed by the cation.
E) the ratio of cation to anion is 1:1.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
37
The arrow is pointing to a(n) 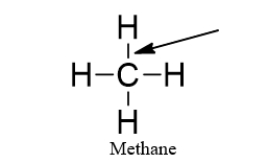
A) triple bond.
B) double bond.
C) single bond.
D) electron.
E) valence shell.

A) triple bond.
B) double bond.
C) single bond.
D) electron.
E) valence shell.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
38
What is the most likely arrangement of the atoms in chloroethylene (C2H3Cl)? 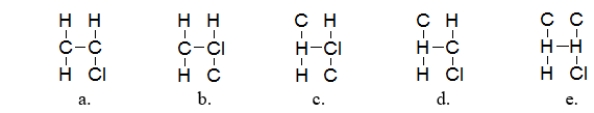
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following is NOT a covalent compound?
A) DNA
B) RNA
C) proteins
D) carbohydrates
E) sodium phosphate
A) DNA
B) RNA
C) proteins
D) carbohydrates
E) sodium phosphate

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
40
The name of Al3+ is ____________.
A) aluminide
B) aluminum ion
C) aluminum three
D) alide
E) trialuminum
A) aluminide
B) aluminum ion
C) aluminum three
D) alide
E) trialuminum

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
41
What is the name of CaCO3, a common calcium supplement and antacid?
A) calcium carbon trioxide
B) calcium bicarbonate
C) calcium carbonate
D) calcium cyanide
E) calcium chlorite
A) calcium carbon trioxide
B) calcium bicarbonate
C) calcium carbonate
D) calcium cyanide
E) calcium chlorite

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
42
What ionic compound has the formula Na2O?
A) disodium oxide
B) sodium oxide
C) sodium dioxide
D) sodium oxygen
E) disodium oxygen
A) disodium oxide
B) sodium oxide
C) sodium dioxide
D) sodium oxygen
E) disodium oxygen

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is NOT a covalent compound?
A) HF
B) H2O
C) CO
D) Li2O
E) CS2
A) HF
B) H2O
C) CO
D) Li2O
E) CS2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
44
Below are four compounds.Choose the statement below that BEST describes these four.
CH2O Ti2O3 MgO H2O
I II III IV
A) All of these are ionic compounds.
B) I and II are covalent molecules, whereas III and IV are ionic.
C) II and III are ionic, whereas I and IV are covalent.
D) I, III, and IV are covalent, whereas II is ionic.
E) All of these are covalent molecules.
CH2O Ti2O3 MgO H2O
I II III IV
A) All of these are ionic compounds.
B) I and II are covalent molecules, whereas III and IV are ionic.
C) II and III are ionic, whereas I and IV are covalent.
D) I, III, and IV are covalent, whereas II is ionic.
E) All of these are covalent molecules.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
45
The conventional way of writing the symbol for an ion of calcium is
A) calcium-18.
B) Ar.
C) Ca-18.
D) Ca2+.
E) Ca2+.
A) calcium-18.
B) Ar.
C) Ca-18.
D) Ca2+.
E) Ca2+.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
46
What is the name of Na2SO3, a preservative in many foods and drinks?
A) sodium sulfite
B) sodium sulfate
C) disodium sulfur trioxide
D) sodium sulfur trioxide
E) disodium trisulfite
A) sodium sulfite
B) sodium sulfate
C) disodium sulfur trioxide
D) sodium sulfur trioxide
E) disodium trisulfite

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
47
In an ionic compound
A) nonmetals share electrons.
B) nonmetals donate electrons to metals.
C) metals are attracted to one another.
D) metals share electrons.
E) cations and anions are attracted to one another.
A) nonmetals share electrons.
B) nonmetals donate electrons to metals.
C) metals are attracted to one another.
D) metals share electrons.
E) cations and anions are attracted to one another.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following statements about the adenosine receptor is NOT true?
A) The receptor only binds adenosine.
B) When adenosine is bound, a signal is sent to the cell to slow activity.
C) The receptor is found embedded in the cell membrane of neurons.
D) It is involved in preparing the brain for sleep.
E) Its normal signaling is blocked when caffeine binds.
A) The receptor only binds adenosine.
B) When adenosine is bound, a signal is sent to the cell to slow activity.
C) The receptor is found embedded in the cell membrane of neurons.
D) It is involved in preparing the brain for sleep.
E) Its normal signaling is blocked when caffeine binds.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
49
Another name for an ionic compound is a(n)________.
A) cation
B) anion
C) ionic bond
D) covalent bond
E) salt
A) cation
B) anion
C) ionic bond
D) covalent bond
E) salt

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
50
A compound contains magnesium and phosphate.What is the formula unit of this compound?
A) Mg3P2
B) MgPO4
C) Mg3(PO4)2
D) Mg2(PO4)3
E) MgHPO4
A) Mg3P2
B) MgPO4
C) Mg3(PO4)2
D) Mg2(PO4)3
E) MgHPO4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
51
How many valence electrons does chlorine have?
A) 5
B) 7
C) 1
D) 5.
E) 6
A) 5
B) 7
C) 1
D) 5.
E) 6

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
52
Below are two charged particles.What will they do when in close proximity to one another? 
A) They will do nothing.
B) The particle on the left will transfer its positive charge to the particle on the right.
C) The particle on the right will transfer its negative charge to the particle on the left.
D) They will attract one another.
E) They will repel one another.

A) They will do nothing.
B) The particle on the left will transfer its positive charge to the particle on the right.
C) The particle on the right will transfer its negative charge to the particle on the left.
D) They will attract one another.
E) They will repel one another.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
53
Which of these structures is a diatomic molecule? 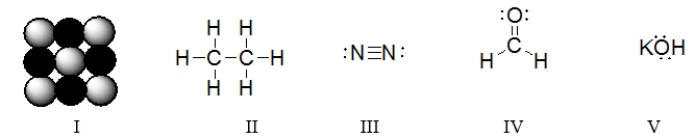
A) I
B) II
C) III
D) IV
E) V
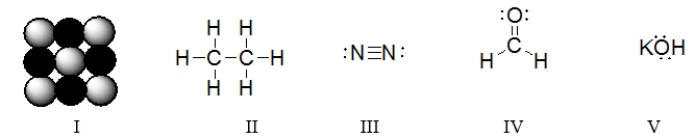
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
54
A polyatomic ion
A) has many atoms, one of which has gained or lost electrons.
B) is a molecule that has gained or lost electrons.
C) has many atoms, one of which has gained or lost protons.
D) is a molecule that has gained or lost protons.
E) has many charges.
A) has many atoms, one of which has gained or lost electrons.
B) is a molecule that has gained or lost electrons.
C) has many atoms, one of which has gained or lost protons.
D) is a molecule that has gained or lost protons.
E) has many charges.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
55
Which statement BEST describes the behavior of period 2 elements in a covalent molecule?
A) Period 2 elements lose electrons.
B) Period 2 elements gain electrons.
C) The nonmetals in period 2 form bonds so that they have a valence shell with eight electrons.
D) The nonmetals in period 2 form bonds so that they have a valence shell with two electrons.
E) Period 2 elements are not alike in any way.
A) Period 2 elements lose electrons.
B) Period 2 elements gain electrons.
C) The nonmetals in period 2 form bonds so that they have a valence shell with eight electrons.
D) The nonmetals in period 2 form bonds so that they have a valence shell with two electrons.
E) Period 2 elements are not alike in any way.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the molecules below contain an atom with an expanded octet? 
A) I and II
B) III only
C) IV only
D) I, II, and III
E) All of these molecules have an expanded octet.

A) I and II
B) III only
C) IV only
D) I, II, and III
E) All of these molecules have an expanded octet.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
57
When a metal atom transfers one or more valence electrons to a nonmetal atom, what are formed?
A) covalent bonds
B) monatomic ions
C) two cations
D) semimetals
E) two anions
A) covalent bonds
B) monatomic ions
C) two cations
D) semimetals
E) two anions

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
58
Nonmetals typically ______, and metals typically ______.
A) donate electrons; accept electrons
B) accept electrons; donate electrons
C) donate protons; accept protons
D) accept protons; donate protons
E) donate neutrons; accept neutrons
A) donate electrons; accept electrons
B) accept electrons; donate electrons
C) donate protons; accept protons
D) accept protons; donate protons
E) donate neutrons; accept neutrons

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
59
An ion that has more electrons than protons is a(n)________.
A) cation
B) anion
C) ionic bond
D) covalent bond
E) salt
A) cation
B) anion
C) ionic bond
D) covalent bond
E) salt

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
60
Below is an illustration of an atom of fluorine.Which arrow points to the part of the atom involved in bonding? 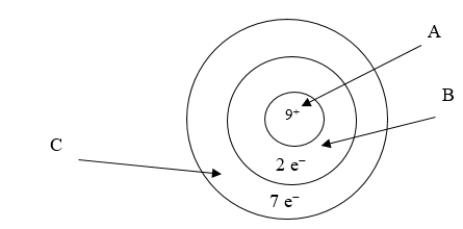
A) A
B) B
C) C
D) Both B and C point to the correct part of the atom.
E) None of the above is involved in bonding.

A) A
B) B
C) C
D) Both B and C point to the correct part of the atom.
E) None of the above is involved in bonding.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
61
An intravenous therapy called Ringer's solutions contains calcium and chloride ions along with other components.What is the charge on calcium in the ionic compound that forms from calcium and chloride ions?
A) +1
B) -1
C) +2
D) -2
E) +3
A) +1
B) -1
C) +2
D) -2
E) +3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
62
Lead(II)is especially harmful to young children because it_________.
A) halts bone growth
B) causes loss of appetite
C) damages the retina
D) damages the heart
E) harms brain development
A) halts bone growth
B) causes loss of appetite
C) damages the retina
D) damages the heart
E) harms brain development

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
63
In general, how many bonds does a nonmetal in period 2 form?
A) the same number as the number of nonbonding electrons in the atom
B) the same number as the number of electrons needed to obtain an octet
C) the same number as the total number of electrons in the atom
D) eight, in accordance with the octet rule
E) It is impossible to predict the number.
A) the same number as the number of nonbonding electrons in the atom
B) the same number as the number of electrons needed to obtain an octet
C) the same number as the total number of electrons in the atom
D) eight, in accordance with the octet rule
E) It is impossible to predict the number.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
64
Magnesium chloride is sometimes administered orally to suppress premature labor.Which of the following is a valid ionic formula for magnesium chloride?
A) Mg2Cl2
B) Mg2Cl
C) MgCl
D) MgCl2
E) None of the above choices are magnesium chloride.
A) Mg2Cl2
B) Mg2Cl
C) MgCl
D) MgCl2
E) None of the above choices are magnesium chloride.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
65
Electrons shared between two atoms is a(n)_______.
A) cation
B) anion
C) ionic bond
D) covalent bond
E) salt
A) cation
B) anion
C) ionic bond
D) covalent bond
E) salt

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
66
What is the ratio of ions in Li2O?
A) 1 Li : 1 O
B) 2 Li : 1 O
C) 2 O : 1 Li
D) 4 O : 5 Li
E) 4 Li : 5 O
A) 1 Li : 1 O
B) 2 Li : 1 O
C) 2 O : 1 Li
D) 4 O : 5 Li
E) 4 Li : 5 O

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
67
The following ionic formula is not valid.Which statement BEST describes what's wrong with this formula? AlCl2
A) It does not include both a nonmetal and a metal.
B) The ratio of cations to anions is not 1:1.
C) The anion is written first, followed by the cation.
D) This compound does not exist in nature.
E) The charges do not add up to zero.
A) It does not include both a nonmetal and a metal.
B) The ratio of cations to anions is not 1:1.
C) The anion is written first, followed by the cation.
D) This compound does not exist in nature.
E) The charges do not add up to zero.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
68
What is the charge on each chloride ion in an ionic compound composed of calcium and chlorine?
A) +1
B) -1
C) +2
D) -2
E) +3
A) +1
B) -1
C) +2
D) -2
E) +3

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
69
A high level of ______ after 12 hours of fasting indicates diabetes.
A) glucose
B) urea
C) potassium
D) amino acids
E) electrolytes
A) glucose
B) urea
C) potassium
D) amino acids
E) electrolytes

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
70
What are the charges on the ions in Li2O?
A) Li has a -1 charge, and O has a +1 charge.
B) Li has a +1 charge, and O has a -1 charge.
C) Li has a -1 charge, and O has a +2 charge.
D) Li has a +1 charge, and O has a -2 charge.
E) Neither lithium nor oxygen is charged.
A) Li has a -1 charge, and O has a +1 charge.
B) Li has a +1 charge, and O has a -1 charge.
C) Li has a -1 charge, and O has a +2 charge.
D) Li has a +1 charge, and O has a -2 charge.
E) Neither lithium nor oxygen is charged.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
71
Below are two ions (not drawn to scale).What will they do when in close proximity to one another? 
A) They will attract one another, forming an ionic compound.
B) They will attract one another, forming a covalent bond.
C) The sodium ion will transfer a proton to the chloride ion.
D) The chloride ion will transfer an electron to the sodium ion.
E) They will do nothing.

A) They will attract one another, forming an ionic compound.
B) They will attract one another, forming a covalent bond.
C) The sodium ion will transfer a proton to the chloride ion.
D) The chloride ion will transfer an electron to the sodium ion.
E) They will do nothing.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following elements might have an expanded octet in a covalent molecule?
A) No elements have an expanded octet.
B) Atoms in period 2 might have an expanded octet.
C) Atoms in period 3 might have an expanded octet.
D) Any of the atoms in group 2 might have an expanded octet.
E) Any of the atoms in group 3 might have an expanded octet.
A) No elements have an expanded octet.
B) Atoms in period 2 might have an expanded octet.
C) Atoms in period 3 might have an expanded octet.
D) Any of the atoms in group 2 might have an expanded octet.
E) Any of the atoms in group 3 might have an expanded octet.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
73
In which of the structures are six electrons being shared between two atoms? 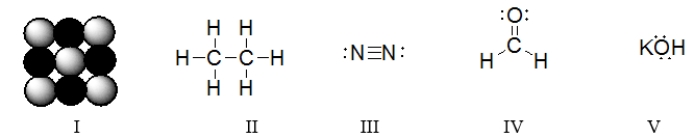
A) I
B) II
C) III
D) IV
E) V
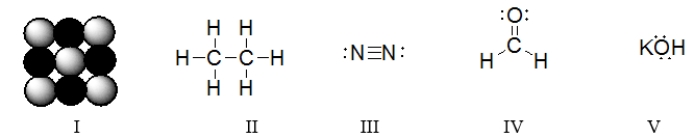
A) I
B) II
C) III
D) IV
E) V

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
74
An ion of cobalt has 25 electrons total.What is the charge of the cobalt ion?
A) +2
B) +1
C) It is neutral.
D) -1
E) -2
A) +2
B) +1
C) It is neutral.
D) -1
E) -2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
75
Which of these structures represent ionic compounds? 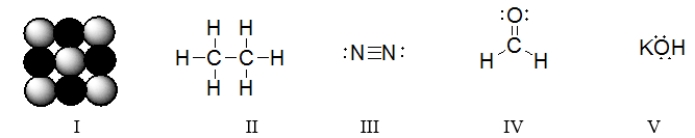
A) I and III
B) I and IV
C) IV and V
D) II and IV
E) I only
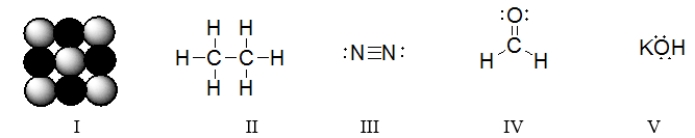
A) I and III
B) I and IV
C) IV and V
D) II and IV
E) I only

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
76
An electrolyte is a substance that
A) dissolves in water.
B) does not dissolve in water.
C) hydrogen bonds with water.
D) produces ions when dissolved in water.
E) is an excellent resistor.
A) dissolves in water.
B) does not dissolve in water.
C) hydrogen bonds with water.
D) produces ions when dissolved in water.
E) is an excellent resistor.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
77
An ion of fluorine has ten electrons total.What is the charge of the fluorine ion?
A) +2
B) +1
C) It is neutral.
D) -1
E) -2
A) +2
B) +1
C) It is neutral.
D) -1
E) -2

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
78
A magnesium ion has the same electron configuration as _______.
A) No other atom or element has the same configuration.
B) sodium
C) neon
D) fluorine
E) aluminum
A) No other atom or element has the same configuration.
B) sodium
C) neon
D) fluorine
E) aluminum

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the structures contains a double bond? 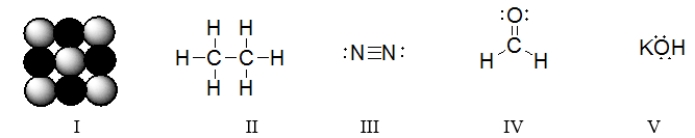
A) I
B) II
C) III
D) IV
E) III and IV
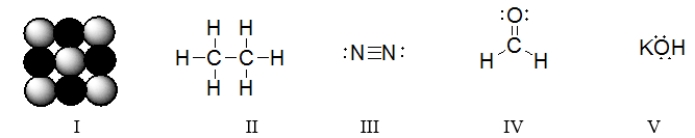
A) I
B) II
C) III
D) IV
E) III and IV

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following ions does NOT have the same number of valence electrons as neon?
A) fluoride ion
B) calcium ion
C) magnesium ion
D) oxide ion
E) sodium ion
A) fluoride ion
B) calcium ion
C) magnesium ion
D) oxide ion
E) sodium ion

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck


