Exam 3: Ionic and Covalent Compounds
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Which of the following is the formula for an ionic compound called iron(II)oxide?
Free
(Multiple Choice)
4.8/5  (34)
(34)
Correct Answer:
C
Which statement BEST describes the behavior of period 2 elements in a covalent molecule?
Free
(Multiple Choice)
4.8/5  (29)
(29)
Correct Answer:
C
How many multiple bonds are in the chloroethylene molecule (C2H3Cl)?
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
B
Below are four compounds.Choose the statement below that BEST describes these four.
CH2O Ti2O3 MgO H2O
I II III IV
(Multiple Choice)
4.9/5  (40)
(40)
A high level of ______ after 12 hours of fasting indicates diabetes.
(Multiple Choice)
4.8/5  (30)
(30)
What is the total number of valence electrons in the chloroethylene molecule (C2H3Cl)?
(Multiple Choice)
4.9/5  (33)
(33)
Which of these structures has two nonbonding pairs of electrons? 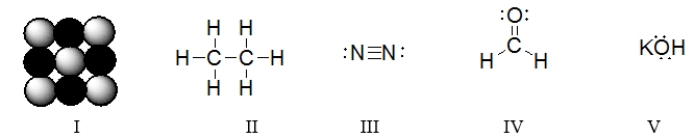
(Multiple Choice)
4.8/5  (39)
(39)
When a metal atom transfers one or more valence electrons to a nonmetal atom, what are formed?
(Multiple Choice)
4.7/5  (40)
(40)
Below is an illustration of an atom of fluorine.Which arrow points to the part of the atom involved in bonding? 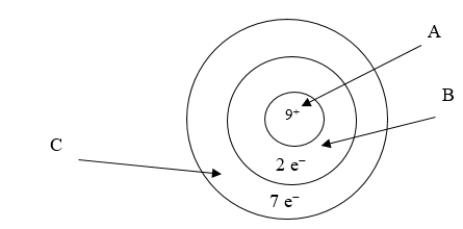
(Multiple Choice)
4.8/5  (38)
(38)
What is the most likely arrangement of the atoms in chloroethylene (C2H3Cl)? 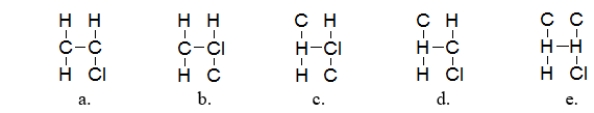
(Multiple Choice)
4.9/5  (42)
(42)
In general, how do elements in group 6A behave when they are a part of a covalent bond?
(Multiple Choice)
4.8/5  (31)
(31)
An intravenous therapy called Ringer's solutions contains calcium and chloride ions along with other components.What is the charge on calcium in the ionic compound that forms from calcium and chloride ions?
(Multiple Choice)
4.9/5  (36)
(36)
An ion of fluorine has ten electrons total.What is the charge of the fluorine ion?
(Multiple Choice)
4.8/5  (32)
(32)
Calcium carbonate has the formula CaCO3.What is the charge on the polyatomic carbonate ion?
(Multiple Choice)
4.9/5  (37)
(37)
Showing 1 - 20 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)