Deck 4: Molecular Geometry, Polarity, and Intermolecular
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/86
Play
Full screen (f)
Deck 4: Molecular Geometry, Polarity, and Intermolecular
1
Propane is a fuel commonly used in barbeques and to heat homes.It has the structure shown below.Does propane have a permanent dipole? 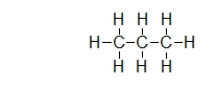
A) Yes.The carbon is partially negative, and the hydrogen is partially positive.
B) Yes.The carbon is partially positive, and the hydrogen is partially negative.
C) Yes.One side of the molecule is always partially positive, and the other side is partially negative.
D) No.Propane has a temporary dipole.
E) No.Propane never displays any dipole at all.

A) Yes.The carbon is partially negative, and the hydrogen is partially positive.
B) Yes.The carbon is partially positive, and the hydrogen is partially negative.
C) Yes.One side of the molecule is always partially positive, and the other side is partially negative.
D) No.Propane has a temporary dipole.
E) No.Propane never displays any dipole at all.
No.Propane has a temporary dipole.
2
An atom in a molecule has one lone pair and three atoms bonded to it.What is the molecular geometry of this atom?
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
trigonal pyramidal
3
The electronegativity difference between C and O is ____ , and therefore, the C-O bond is a(n)____ bond.
A) 1.0; nonpolar covalent
B) 6.0; polar covalent
C) -1.0; ionic
D) 6.0; nonpolar covalent
E) 1.0; polar covalent
A) 1.0; nonpolar covalent
B) 6.0; polar covalent
C) -1.0; ionic
D) 6.0; nonpolar covalent
E) 1.0; polar covalent
1.0; polar covalent
4
What is the electron geometry of the carbon in dichloromethane (CH2Cl2)?
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
5
The electronegativity difference between K and Cl is ____, and therefore, the Mg-Cl bond is a(n)____ bond.
A) 2.2; polar covalent
B) -2.2; nonpolar covalent
C) 3.8; ionic
D) -3.8; nonpolar covalent
E) 2.2; ionic
A) 2.2; polar covalent
B) -2.2; nonpolar covalent
C) 3.8; ionic
D) -3.8; nonpolar covalent
E) 2.2; ionic

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
6
What is the electron geometry of the groups around the nitrogen atom indicated with arrow II? 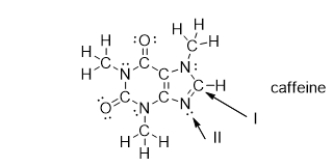
A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) linear

A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) linear

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
7
What is the molecular geometry of the carbon in dichloromethane (CH2Cl2)?
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
8
Which figure BEST illustrates how two molecules of formaldehyde (CH2O)interact? 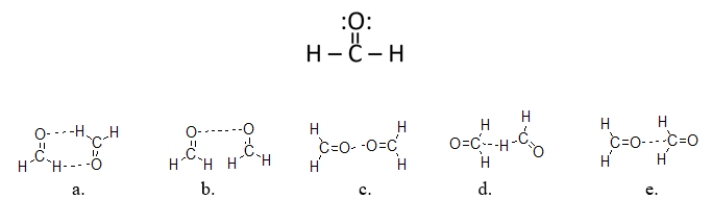
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
9
Electrostatic interactions between positive and negative ions are called _______.
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
10
An atom, X, has a tetrahedral electron geometry but a trigonal pyramidal molecular geometry.How many atoms is atom X bonded to?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following covalent compounds is polar? 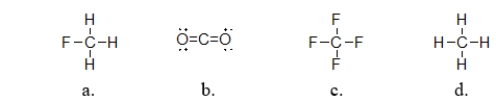
A) structure a
B) structure b
C) structure c
D) structure d
E) all of these molecules are polar.
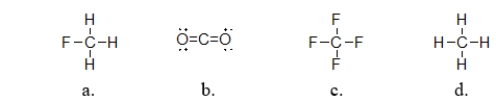
A) structure a
B) structure b
C) structure c
D) structure d
E) all of these molecules are polar.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
12
Chloroform (CHCl3)is an anesthetic and is also used in the synthesis of ozone-damaging refrigerants called CFCs.What is the correct Lewis dot structure for chloroform? 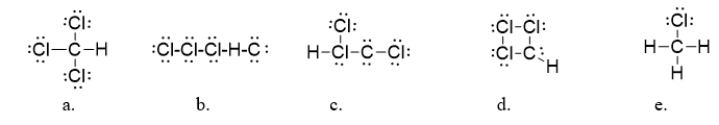
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e
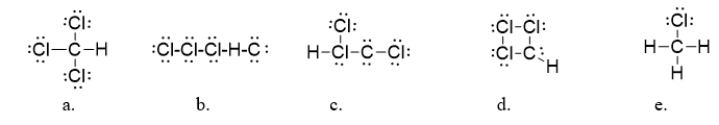
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
13
When determining the shape of a molecule, it is necessary to count electron groups.Which of the following is an electron group?
A) a single bond
B) a double bond
C) a triple bond
D) a nonbonding pair of electrons
E) All of these are electron groups.
A) a single bond
B) a double bond
C) a triple bond
D) a nonbonding pair of electrons
E) All of these are electron groups.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
14
Nitrogen trichloride, once used as a bleaching agent, causes neurological disorder and was banned in 1949.Which of the following is a correct Lewis structure for nitrogen trichloride (NCl3)? 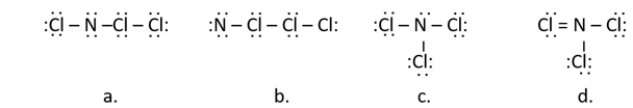
A) structure a
B) structure b
C) structure c
D) structure d
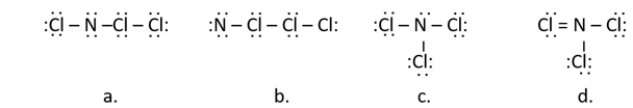
A) structure a
B) structure b
C) structure c
D) structure d

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
15
What is the molecular geometry of the atoms bonded to oxygen in methanol, shown below? 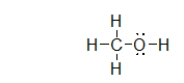
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
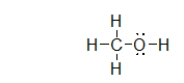
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
16
Does propane (shown)or octane (C8H18)exhibit stronger dispersion forces? 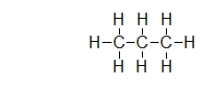
A) Propane.The smaller molecules can get closer to one another.
B) Propane.It has a larger permanent dipole.
C) Octane.It has a larger permanent dipole.
D) Octane.It has more electrons.
E) They both have the same amount of forces.

A) Propane.The smaller molecules can get closer to one another.
B) Propane.It has a larger permanent dipole.
C) Octane.It has a larger permanent dipole.
D) Octane.It has more electrons.
E) They both have the same amount of forces.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
17
How many electron groups do the carbon atoms in ethylene (C2H4)have?
A) They both have one group.
B) They both have two groups.
C) They both have three groups.
D) They both have four groups.
E) One has two groups, and the other has four groups.
A) They both have one group.
B) They both have two groups.
C) They both have three groups.
D) They both have four groups.
E) One has two groups, and the other has four groups.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the molecules below exhibits hydrogen bonding forces between like molecules? 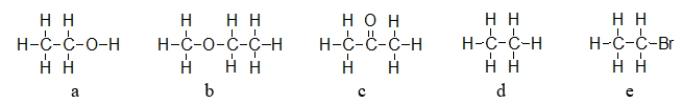
A) a only
B) a and b
C) a, b, and c
D) a, b, c, and d
E) e only

A) a only
B) a and b
C) a, b, and c
D) a, b, c, and d
E) e only

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
19
How many nonbonding pairs are on the carbon in dichloromethane (CH2Cl2)?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following bonds is nonpolar?
A) C-O
B) O-F
C) C-F
D) C-H
E) H-F
A) C-O
B) O-F
C) C-F
D) C-H
E) H-F

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
21
The electronegativity difference between C and H is ____, and therefore, the C-H bond is a(n)____ bond.
A) 0.4; nonpolar covalent
B) 0.4; polar covalent
C) 4.6; ionic
D) -0.4; nonpolar covalent
E) 4.6; polar covalent
A) 0.4; nonpolar covalent
B) 0.4; polar covalent
C) 4.6; ionic
D) -0.4; nonpolar covalent
E) 4.6; polar covalent

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
22
Does formaldehyde have a permanent dipole? 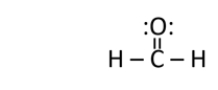
A) Yes.The carbon is partially negative, and the oxygen is partially positive.
B) Yes.The carbon is partially negative, and the hydrogen is partially positive.
C) Yes.The carbon is partially positive, and the oxygen is partially negative.
D) Yes.The carbon is partially positive, and the hydrogen is partially negative.
E) No.Formaldehyde only has a temporary dipole.
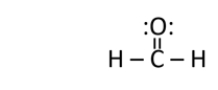
A) Yes.The carbon is partially negative, and the oxygen is partially positive.
B) Yes.The carbon is partially negative, and the hydrogen is partially positive.
C) Yes.The carbon is partially positive, and the oxygen is partially negative.
D) Yes.The carbon is partially positive, and the hydrogen is partially negative.
E) No.Formaldehyde only has a temporary dipole.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
23
What is the molecular geometry of the atoms bonded to the nitrogen atom indicated with arrow II? 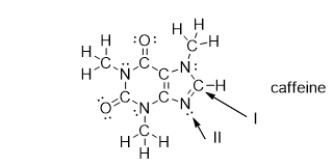
A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) linear

A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) linear

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
24
Which element is the MOST electronegative?
A) fluorine
B) bromine
C) chlorine
D) iodine
E) boron
A) fluorine
B) bromine
C) chlorine
D) iodine
E) boron

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
25
A polar molecule is one that has
A) one large atom and one small atom.
B) an even distribution of charge.
C) a positive or negative charge.
D) a more positive side and a more negative side.
E) only polar atoms.
A) one large atom and one small atom.
B) an even distribution of charge.
C) a positive or negative charge.
D) a more positive side and a more negative side.
E) only polar atoms.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
26
In molecules with permanent dipoles, _______ are the attraction of the positive end of a dipole on one molecule with the negative end of the dipole on another molecule.
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
27
What is the strongest type of intermolecular force of attraction that a caffeine molecule could form with other caffeine molecules? 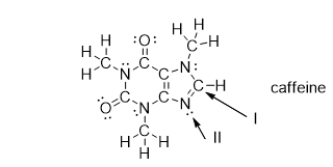
A) dispersion forces
B) nonpolar covalent bonds
C) hydrogen bonds
D) covalent bonds
E) dipole-dipole interactions

A) dispersion forces
B) nonpolar covalent bonds
C) hydrogen bonds
D) covalent bonds
E) dipole-dipole interactions

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
28
How does VSEPR theory explain the electron group geometry of a molecule?
A) The bonds arrange themselves at 90º angles.
B) The groups are arranged as far away from each other as possible.
C) The groups are as close to each other as possible.
D) The bonds are close together but far away from the nonbonding pairs.
E) None of these answers explains VSEPR theory.
A) The bonds arrange themselves at 90º angles.
B) The groups are arranged as far away from each other as possible.
C) The groups are as close to each other as possible.
D) The bonds are close together but far away from the nonbonding pairs.
E) None of these answers explains VSEPR theory.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
29
Why do electron groups around a central atom arrange themselves as far apart from one another as possible, while still remaining attached to the central atom?
A) Actually, electrons don't do this, but atoms do.
B) The like charges of the electrons attract each other.
C) The protons direct the electrons to do this.
D) The like charges of the electrons repel each other.
E) There is no reason for this arrangement; it is just observed to be that way.
A) Actually, electrons don't do this, but atoms do.
B) The like charges of the electrons attract each other.
C) The protons direct the electrons to do this.
D) The like charges of the electrons repel each other.
E) There is no reason for this arrangement; it is just observed to be that way.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
30
What is the strongest type of intermolecular force of attraction that a caffeine molecule could form with water? 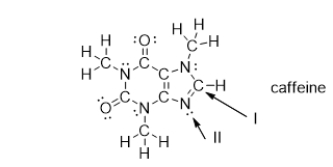
A) dispersion forces
B) nonpolar covalent bonds
C) hydrogen bonds
D) covalent bonds
E) dipole-dipole interactions

A) dispersion forces
B) nonpolar covalent bonds
C) hydrogen bonds
D) covalent bonds
E) dipole-dipole interactions

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
31
Atom X in a molecule has tetrahedral electron geometry but a bent molecular geometry.Which of the following describes the identity of atom X?
A) Atom X is the oxygen in a molecule of H2O.
B) Atom X is the nitrogen in a molecule of NH3.
C) Atom X is the hydrogen in a molecule of CH4.
D) Atom X is the carbon in a molecule of CH4.
E) Atom X could be any of these atoms.
A) Atom X is the oxygen in a molecule of H2O.
B) Atom X is the nitrogen in a molecule of NH3.
C) Atom X is the hydrogen in a molecule of CH4.
D) Atom X is the carbon in a molecule of CH4.
E) Atom X could be any of these atoms.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
32
Is chloroform (CHCl3)a polar molecule?
A) No.All polarities cancel out.
B) Yes.All molecules with polar bonds are polar.
C) No.The bonds in chloroform are not polar.
D) Yes.The polarities do not cancel out.
E) No.Carbon compounds cannot be polar.
A) No.All polarities cancel out.
B) Yes.All molecules with polar bonds are polar.
C) No.The bonds in chloroform are not polar.
D) Yes.The polarities do not cancel out.
E) No.Carbon compounds cannot be polar.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
33
Does ethanol have a permanent dipole? 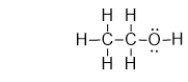
A) Yes.The carbon is partially negative, and the oxygen is partially positive.
B) Yes.The carbon is partially negative, and the hydrogen is partially positive.
C) Yes.The hydrogen is partially positive, and the oxygen is partially negative.
D) Yes.The oxygen is partially positive, and the hydrogen is partially negative.
E) No.Ethanol only has a temporary dipole.

A) Yes.The carbon is partially negative, and the oxygen is partially positive.
B) Yes.The carbon is partially negative, and the hydrogen is partially positive.
C) Yes.The hydrogen is partially positive, and the oxygen is partially negative.
D) Yes.The oxygen is partially positive, and the hydrogen is partially negative.
E) No.Ethanol only has a temporary dipole.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
34
What is the angle between groups of electrons for an atom that has a linear electron geometry?
A) 90°
B) 120°
C) 180°
D) 109.5°
E) 360°
A) 90°
B) 120°
C) 180°
D) 109.5°
E) 360°

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
35
Tamoxifen has some key similarities to estradiol.Which of the following statements describes one of the similarities?
A) Tamoxifen has the same structure as estradiol.
B) Tamoxifen has the same number of atoms as estradiol.
C) Tamoxifen performs the same function in the cell as estradiol.
D) Tamoxifen binds to the same receptor as estradiol.
E) Tamoxifen deforms the shape of the estrogen receptor in the same way as estradiol.
A) Tamoxifen has the same structure as estradiol.
B) Tamoxifen has the same number of atoms as estradiol.
C) Tamoxifen performs the same function in the cell as estradiol.
D) Tamoxifen binds to the same receptor as estradiol.
E) Tamoxifen deforms the shape of the estrogen receptor in the same way as estradiol.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
36
Which element is the LEAST electronegative?
A) potassium
B) hydrogen
C) fluorine
D) carbon
E) oxygen
A) potassium
B) hydrogen
C) fluorine
D) carbon
E) oxygen

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
37
An atom in a molecule has two lone pairs and two atoms bonded to it.What is the molecular geometry of this atom?
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following steps is done first to determine the molecular geometry of dichloromethane (CH2Cl2)?
A) Calculate the molecular weight.
B) Look up the bond angle of a H-C-H bond.
C) Determine the electron geometry.
D) Construct the Lewis structure.
E) The molecular geometry can be determined without doing any prior steps.
A) Calculate the molecular weight.
B) Look up the bond angle of a H-C-H bond.
C) Determine the electron geometry.
D) Construct the Lewis structure.
E) The molecular geometry can be determined without doing any prior steps.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
39
Which type(s)of molecular model illustrate the three-dimensional shape of a molecule?
A) ball-and-stick
B) Lewis dot structure
C) space-filling
D) Lewis dot and space-filling
E) ball-and-stick and space-filling
A) ball-and-stick
B) Lewis dot structure
C) space-filling
D) Lewis dot and space-filling
E) ball-and-stick and space-filling

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
40
Which elements have the highest electronegativity and why?
A) Nonmetals.They accept electrons in order to attain an octet.
B) Metals.They accept electrons in order to attain an octet.
C) Nonmetals.They donate electrons in order to attain an octet.
D) Metals.They donate electrons in order to attain an octet.
E) Transition metals.They can have different charges.
A) Nonmetals.They accept electrons in order to attain an octet.
B) Metals.They accept electrons in order to attain an octet.
C) Nonmetals.They donate electrons in order to attain an octet.
D) Metals.They donate electrons in order to attain an octet.
E) Transition metals.They can have different charges.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following molecules contains polar bonds but is nonpolar overall?
A) Cl2
B) CF4
C) H2O
D) N2
E) NH3
A) Cl2
B) CF4
C) H2O
D) N2
E) NH3

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
42
What is the molecular geometry of each carbon in ethylene (C2H4)?
A) They are both linear.
B) They are both trigonal planar.
C) They are both bent.
D) One is bent, and the other is trigonal planar.
E) One is bent, and the other is tetrahedral.
A) They are both linear.
B) They are both trigonal planar.
C) They are both bent.
D) One is bent, and the other is trigonal planar.
E) One is bent, and the other is tetrahedral.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
43
Formaldehyde is used as a preservative and disinfectant.It has the structure shown below.What is the strongest type of intermolecular interaction attracting molecules of formaldehyde together? 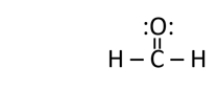
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces
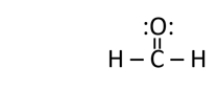
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
44
An atom in a molecule has a trigonal planar molecular geometry and a formula of MX3.What is the angle between the atoms in this molecule?
A) 360º
B) 180º
C) 120º
D) 109.5º
E) 90º
A) 360º
B) 180º
C) 120º
D) 109.5º
E) 90º

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
45
Which bonds in caffeine are polar covalent bonds? 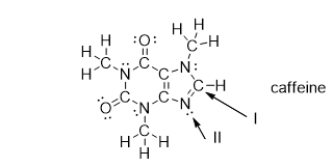
A) All the bonds are polar covalent.
B) the C-N bonds only
C) the C=C, C-C and C-H bonds
D) the C-N, C=O, and C=N bonds
E) the O-H bonds

A) All the bonds are polar covalent.
B) the C-N bonds only
C) the C=C, C-C and C-H bonds
D) the C-N, C=O, and C=N bonds
E) the O-H bonds

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
46
How does estradiol stimulate the growth of breast cancer cells?
A) Estradiol is a toxin, and all toxins stimulate the growth and spread of cancer.
B) Estradiol is a damaged estrogen that stimulates the growth of damaged cell.
C) Estradiol binds to breast cancer cell receptors as well as receptors found in other parts of the body.
D) Once a woman reaches adulthood, estradiol has no purpose in the body and so it causes breast cancer.
E) All of the above statements about estradiol are true.
A) Estradiol is a toxin, and all toxins stimulate the growth and spread of cancer.
B) Estradiol is a damaged estrogen that stimulates the growth of damaged cell.
C) Estradiol binds to breast cancer cell receptors as well as receptors found in other parts of the body.
D) Once a woman reaches adulthood, estradiol has no purpose in the body and so it causes breast cancer.
E) All of the above statements about estradiol are true.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
47
Which BEST describes your reasoning in answering the previous question? 
A) Ethanol is nonpolar.
B) Ethanol has polar bonds.
C) Ethanol contains an O-H bond.
D) Ethanol forms ions in solutions.
E) Ethanol does not contain any metals.

A) Ethanol is nonpolar.
B) Ethanol has polar bonds.
C) Ethanol contains an O-H bond.
D) Ethanol forms ions in solutions.
E) Ethanol does not contain any metals.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
48
The only interactions between two or more molecules of a nonpolar material such as methane (CH4)are _______ because these molecules do not have permanent dipoles.
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
49
What is the angle between groups of electrons for an atom that has a tetrahedral electron geometry?
A) 90°
B) 120°
C) 180°
D) 109.5°
E) 360°
A) 90°
B) 120°
C) 180°
D) 109.5°
E) 360°

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
50
What is the strongest type of intermolecular interaction attracting molecules of propane together? 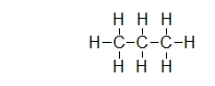
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces

A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
51
Electronegativity is a measure of an atom's ability to
A) become an anion.
B) ionize.
C) draw electrons to itself in a covalent bond.
D) accept an electron from a metal.
E) donate an electron to a nonmetal.
A) become an anion.
B) ionize.
C) draw electrons to itself in a covalent bond.
D) accept an electron from a metal.
E) donate an electron to a nonmetal.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
52
Which figure BEST illustrates how two molecules of ethanol interact? 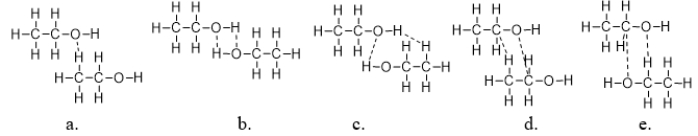
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
53
Which bond is correctly labeled with a dipole arrow? 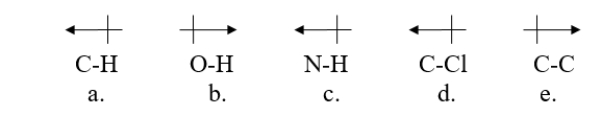
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following interactions is the strongest?
A) dipole-dipole forces
B) hydrogen bonding forces
C) dispersion forces
D) covalent bond
E) All of the above interactions have the same strength.
A) dipole-dipole forces
B) hydrogen bonding forces
C) dispersion forces
D) covalent bond
E) All of the above interactions have the same strength.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the molecules below does NOT have a permanent dipole? 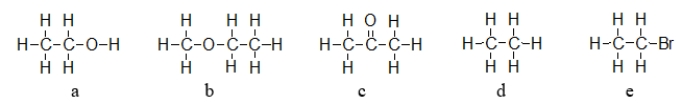
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
56
What is the purpose of Valence Shell Electron Pair Repulsion (VSEPR)theory?
A) It predicts which atom is the central atom in a Lewis structure.
B) It identifies the lone pair and valence electrons in a molecule.
C) It determines the shape of a molecule from the Lewis structure.
D) It determines the shape of a molecule from a molecular model.
E) It determines the arrangement of valence electrons in an atom.
A) It predicts which atom is the central atom in a Lewis structure.
B) It identifies the lone pair and valence electrons in a molecule.
C) It determines the shape of a molecule from the Lewis structure.
D) It determines the shape of a molecule from a molecular model.
E) It determines the arrangement of valence electrons in an atom.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
57
Ethanol is an alcohol found in wine and beer, and it is also used as a fuel.It has the structure shown below.What is the strongest type of intermolecular interaction attracting molecules of ethanol together? 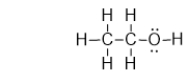
A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces

A) ionic bonding
B) covalent bonding
C) dispersion forces
D) dipole-dipole forces
E) hydrogen bonding forces

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
58
In addition to single bonds, which of the following electron groups are on the nitrogen of nitrogen trichloride (NCl3)?
A) a double bond
B) a triple bond
C) a single electron
D) a lone pair of electrons
E) None of the above electron groups are on the nitrogen.
A) a double bond
B) a triple bond
C) a single electron
D) a lone pair of electrons
E) None of the above electron groups are on the nitrogen.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
59
Which molecule below exhibits the strongest intermolecular forces of attraction? 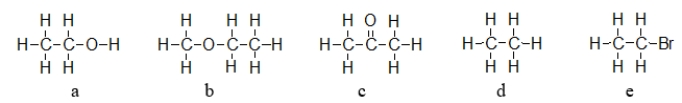
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
60
What is the electron geometry of the nitrogen atom in nitrogen trichloride (NCl3)?
A) trigonal planar
B) bent
C) trigonal pyramidal
D) tetrahedral
E) linear
A) trigonal planar
B) bent
C) trigonal pyramidal
D) tetrahedral
E) linear

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
61
What is the molecular geometry of the carbon in formaldehyde, shown below? 
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
62
Ethylene is used as a starting material in making plastics.It has a molecular formula of C2H4.Which of the following structures is the correct Lewis structure for ethylene? 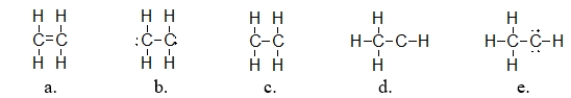
A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

A) structure a
B) structure b
C) structure c
D) structure d
E) structure e

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
63
What is the electron geometry of the carbon in formaldehyde, shown below? 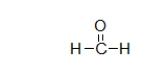
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
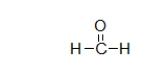
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
64
Which molecules exhibit the strongest intermolecular forces of attraction? 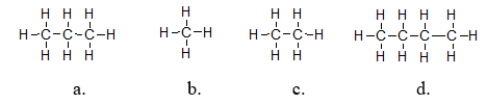
A) structure a
B) structure b
C) structure c
D) structure d
E) They all have the same strength of intermolecular force because they are all nonpolar molecules
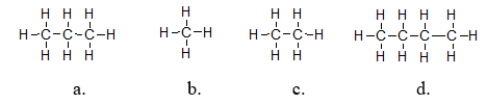
A) structure a
B) structure b
C) structure c
D) structure d
E) They all have the same strength of intermolecular force because they are all nonpolar molecules

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
65
What is the molecular geometry of carbon in chloroform (CHCl3)?
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
66
What is the electron geometry of the carbon in chloroform (CHCl3)?
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
E) tetrahedral

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
67
Antiestrogens are one type of molecule that can be used to treat breast cancer.Which of the following characteristics should be included in the design of a novel antiestrogen?
A) The molecule should bind to the estrogen receptor on breast cancer cells.
B) The molecule should not interfere with the normal role of estrogen.
C) The molecule should prevent the activation of genes in breast cancer cells.
D) The molecule should not have negative side effects.
E) All of the above are design considerations.
A) The molecule should bind to the estrogen receptor on breast cancer cells.
B) The molecule should not interfere with the normal role of estrogen.
C) The molecule should prevent the activation of genes in breast cancer cells.
D) The molecule should not have negative side effects.
E) All of the above are design considerations.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
68
In addition to single bonds, which of the following electron groups do the carbon atoms in ethylene (C2H4)have?
A) a double bond
B) a triple bond
C) a single electron
D) a lone pair of electrons
E) None of the above electron groups is correct.
A) a double bond
B) a triple bond
C) a single electron
D) a lone pair of electrons
E) None of the above electron groups is correct.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
69
Which of these molecules exhibits dipole-dipole forces but cannot hydrogen bond with like molecules? 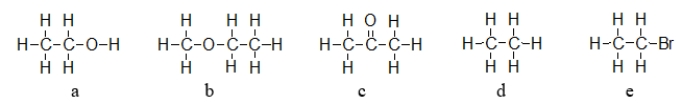
A) b only
B) a, b, and c
C) b, c, and e
D) a, b, c, and e
E) d only

A) b only
B) a, b, and c
C) b, c, and e
D) a, b, c, and e
E) d only

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following covalent bonds is polar?
A) C-C
B) C-H
C) O-Cl
D) Cl-Cl
E) Mg-Cl
A) C-C
B) C-H
C) O-Cl
D) Cl-Cl
E) Mg-Cl

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
71
Does chloroform (CHCl3)contain polar bonds?
A) Yes.All of the bonds in chloroform are polar.
B) Yes.The C-Cl bond in chloroform is polar.
C) Yes.The C-H bond in chloroform is polar.
D) Yes.All atoms in chloroform are highly electronegative.
E) No.All bonds in chloroform are nonpolar.
A) Yes.All of the bonds in chloroform are polar.
B) Yes.The C-Cl bond in chloroform is polar.
C) Yes.The C-H bond in chloroform is polar.
D) Yes.All atoms in chloroform are highly electronegative.
E) No.All bonds in chloroform are nonpolar.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
72
What is the H-C-H bond angle of each carbon in ethylene (C2H4)?
A) Both are 180º.
B) Both are 120º.
C) Both are 109.5º.
D) One is 180º, and the other is 109.5º.
E) One is 120º, and the other is 109.5º.
A) Both are 180º.
B) Both are 120º.
C) Both are 109.5º.
D) One is 180º, and the other is 109.5º.
E) One is 120º, and the other is 109.5º.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the bonds in the following molecule are polar covalent? 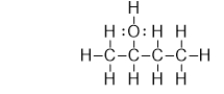
A) C-H and O-H only
B) C-O and O-H only
C) C-O only
D) C-C and C-O
E) O-H only

A) C-H and O-H only
B) C-O and O-H only
C) C-O only
D) C-C and C-O
E) O-H only

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
74
How many electron groups are found on the nitrogen atom of nitrogen trichloride (NCl3)?
A) one
B) two
C) three
D) four
E) five
A) one
B) two
C) three
D) four
E) five

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
75
Which element is the MOST electronegative?
A) nitrogen
B) carbon
C) oxygen
D) sulfur
E) selenium
A) nitrogen
B) carbon
C) oxygen
D) sulfur
E) selenium

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
76
______ is the sharing of electrons between two atoms and is much stronger than intermolecular forces of attraction.
A) Ionic bonding
B) Covalent bonding
C) Dispersion forces
D) Dipole-dipole forces
E) Hydrogen bonding forces
A) Ionic bonding
B) Covalent bonding
C) Dispersion forces
D) Dipole-dipole forces
E) Hydrogen bonding forces

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
77
How many atoms are bonded to the carbon in dichloromethane (CH2Cl2)?
A) 0
B) 1
C) 2
D) 3
E) 4
A) 0
B) 1
C) 2
D) 3
E) 4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
78
What is the angle between groups of electrons for an atom that has a trigonal planar electron geometry?
A) 90°
B) 120°
C) 180°
D) 109.5°
E) 360°
A) 90°
B) 120°
C) 180°
D) 109.5°
E) 360°

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the bonds in the following molecule are polar covalent? 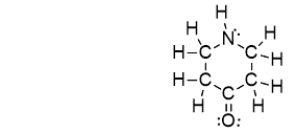
A) C-H only
B) C-N only
C) C=O only
D) C-N and C=O
E) C-N, C=O, and N-H

A) C-H only
B) C-N only
C) C=O only
D) C-N and C=O
E) C-N, C=O, and N-H

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
80
What is the molecular geometry of nitrogen trichloride (NCl3)?
A) trigonal planar
B) bent
C) trigonal pyramidal.
D) nitrogen is tetrahedral
E) linear
A) trigonal planar
B) bent
C) trigonal pyramidal.
D) nitrogen is tetrahedral
E) linear

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck


