Exam 4: Molecular Geometry, Polarity, and Intermolecular
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
In addition to single bonds, which of the following electron groups do the carbon atoms in ethylene (C2H4)have?
Free
(Multiple Choice)
4.7/5  (32)
(32)
Correct Answer:
A
Which of the bonds in the following molecule are polar covalent? 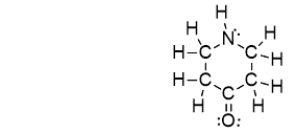
Free
(Multiple Choice)
4.8/5  (33)
(33)
Correct Answer:
E
What is the electron geometry of each carbon in ethylene (C2H4)?
Free
(Multiple Choice)
4.9/5  (34)
(34)
Correct Answer:
B
Ethanol is an alcohol found in wine and beer, and it is also used as a fuel.It has the structure shown below.What is the strongest type of intermolecular interaction attracting molecules of ethanol together? 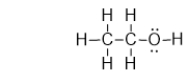
(Multiple Choice)
4.7/5  (39)
(39)
What is the electron geometry of the nitrogen atom in nitrogen trichloride (NCl3)?
(Multiple Choice)
4.9/5  (34)
(34)
The electronegativity difference between C and O is ____ , and therefore, the C-O bond is a(n)____ bond.
(Multiple Choice)
4.8/5  (36)
(36)
What is the molecular geometry of each carbon in ethylene (C2H4)?
(Multiple Choice)
4.9/5  (31)
(31)
What is the molecular geometry of carbon in chloroform (CHCl3)?
(Multiple Choice)
4.8/5  (35)
(35)
The structure below is a Lewis structure of methane.This Lewis structure tells us many things about methane that are useful.However, it also suggests one characteristic of methane that is, in fact, false.What is this one characteristic? 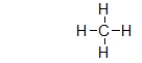
(Multiple Choice)
4.9/5  (38)
(38)
Which molecules exhibit the strongest intermolecular forces of attraction? 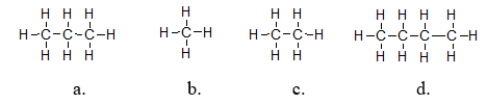
(Multiple Choice)
4.7/5  (37)
(37)
Tamoxifen has some key similarities to estradiol.Which of the following statements describes one of the similarities?
(Multiple Choice)
4.9/5  (32)
(32)
The electronegativity difference between C and H is ____, and therefore, the C-H bond is a(n)____ bond.
(Multiple Choice)
4.8/5  (39)
(39)
The only interactions between two or more molecules of a nonpolar material such as methane (CH4)are _______ because these molecules do not have permanent dipoles.
(Multiple Choice)
4.8/5  (37)
(37)
Chloroform (CHCl3)is an anesthetic and is also used in the synthesis of ozone-damaging refrigerants called CFCs.What is the correct Lewis dot structure for chloroform? 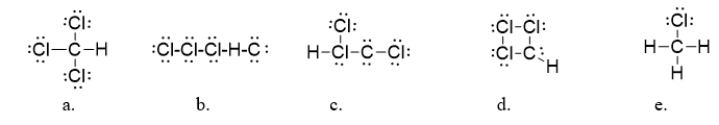
(Multiple Choice)
4.9/5  (30)
(30)
What is the electron geometry of the groups around the nitrogen atom indicated with arrow II? 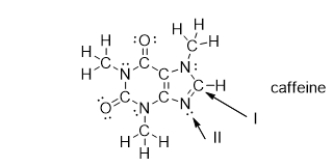
(Multiple Choice)
4.9/5  (38)
(38)
Showing 1 - 20 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)