Deck 2: Atomic Structure and Radioisotopes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/107
Play
Full screen (f)
Deck 2: Atomic Structure and Radioisotopes
1
The elements numbered 21 through 30 are examples of _______.
A) transition metals
B) noble gases
C) alkali earth metals
D) alkali metals
E) halogens
A) transition metals
B) noble gases
C) alkali earth metals
D) alkali metals
E) halogens
transition metals
2
The identity of an element is determined by its number of _____.
A) protons
B) neutrons
C) electrons
D) protons and neutrons
E) protons, neutrons, and electrons
A) protons
B) neutrons
C) electrons
D) protons and neutrons
E) protons, neutrons, and electrons
protons
3
What is the relationship between the energy and the wavelength of light?
A) They are proportional.
B) They are inversely proportional.
C) As one increases, so does the other, but not in a linear way.
D) As one increases, the other decreases, but not in a predictable way.
E) They are unrelated in any way.
A) They are proportional.
B) They are inversely proportional.
C) As one increases, so does the other, but not in a linear way.
D) As one increases, the other decreases, but not in a predictable way.
E) They are unrelated in any way.
They are inversely proportional.
4
What is the mass number of an atom of oxygen with seven neutrons?
A) 1
B) 7
C) 8
D) 15
E) 15.9994
A) 1
B) 7
C) 8
D) 15
E) 15.9994

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
5
How is an atom in the body changed once it is hit with ionizing radiation?
A) It becomes positively charged.
B) It becomes negatively charged.
C) It becomes a new isotope.
D) It becomes a new element.
E) It falls apart.
A) It becomes positively charged.
B) It becomes negatively charged.
C) It becomes a new isotope.
D) It becomes a new element.
E) It falls apart.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is NOT a building block element?
A) C
B) H
C) O
D) N
E) These are all building block elements.
A) C
B) H
C) O
D) N
E) These are all building block elements.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
7
What type of radiation is emitted when U-235 undergoes radioactive decay? 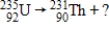
A) alpha particle
B) beta particle
C) positron
D) gamma ray
E) x-ray
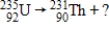
A) alpha particle
B) beta particle
C) positron
D) gamma ray
E) x-ray

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
8
According to the periodic table, the atomic mass of potassium (K)is ______.
A) 4
B) 19
C) 39.10
D) K
E) 2
A) 4
B) 19
C) 39.10
D) K
E) 2

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
9
_____ is most commonly ingested along with salt.
A) Iodine
B) Fluorine
C) Zinc
D) Iron
E) Oxygen
A) Iodine
B) Fluorine
C) Zinc
D) Iron
E) Oxygen

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
10
How is a beta particle different from an electron?
A) They are the same.
B) A beta particle has higher energy than a regular electron
C) A regular electron has higher energy than a beta particle.
D) A beta particle is positively charged.
E) A beta particle is positively charged and higher in energy than a regular electron.
A) They are the same.
B) A beta particle has higher energy than a regular electron
C) A regular electron has higher energy than a beta particle.
D) A beta particle is positively charged.
E) A beta particle is positively charged and higher in energy than a regular electron.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
11
According the graph, what percent of radium-226 remains after three half-lives? 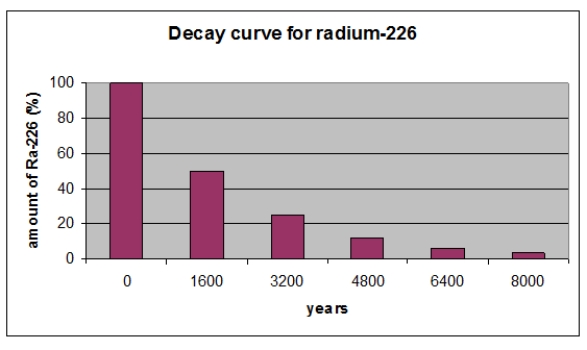
A) 100%
B) 50%
C) 25%
D) 12.5%
E) 6.25%

A) 100%
B) 50%
C) 25%
D) 12.5%
E) 6.25%

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following terms is NOT a characteristic of a metal?
A) malleable
B) a good conductor of heat
C) a good conductor of electricity
D) shiny
E) a gas at room temperature
A) malleable
B) a good conductor of heat
C) a good conductor of electricity
D) shiny
E) a gas at room temperature

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
13
_____ have a negative charge.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following statements about isotopes is FALSE?
A) Isotopes are atoms with the same number of protons but different numbers of neutrons.
B) Most elements naturally have more than one isotope.
C) Isotopes are atoms with the same atomic number but different mass numbers.
D) An isotope with more neutrons will have a greater mass than an isotope with fewer neutrons.
E) Isotopes are present in equal quantities.
A) Isotopes are atoms with the same number of protons but different numbers of neutrons.
B) Most elements naturally have more than one isotope.
C) Isotopes are atoms with the same atomic number but different mass numbers.
D) An isotope with more neutrons will have a greater mass than an isotope with fewer neutrons.
E) Isotopes are present in equal quantities.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
15
What is the atomic number of element X? 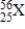
A) 25
B) 56
C) 81
D) 31
E) None of the above values is the atomic number.
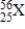
A) 25
B) 56
C) 81
D) 31
E) None of the above values is the atomic number.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
16
According to the periodic table, how many energy levels do the elements in the third row have?
A) 1
B) 2
C) 3
D) 4
E) It depends on the specific element.
A) 1
B) 2
C) 3
D) 4
E) It depends on the specific element.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
17
The number of protons is equal to the _____ in a neutral atom.
A) number of neutrons
B) number of electrons
C) mass number
D) average atomic mass
E) group number
A) number of neutrons
B) number of electrons
C) mass number
D) average atomic mass
E) group number

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is NOT true for the atoms 12C, 13C, and 14C?
A) They all have six electrons.
B) They all have the same mass number.
C) They all have the same atomic number.
D) They are isotopes.
E) They all have six protons.
A) They all have six electrons.
B) They all have the same mass number.
C) They all have the same atomic number.
D) They are isotopes.
E) They all have six protons.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
19
What is the identity of the missing daughter nuclide in the following nuclear reaction? 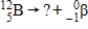
A) beryllium-11
B) beryllium-12
C) beryllium-13
D) carbon-12
E) carbon-13
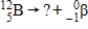
A) beryllium-11
B) beryllium-12
C) beryllium-13
D) carbon-12
E) carbon-13

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
20
What fraction of the electromagnetic spectrum is visible to humans?
A) about two-thirds
B) half
C) about one-quarter
D) a very small fraction
E) none
A) about two-thirds
B) half
C) about one-quarter
D) a very small fraction
E) none

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
21
Which electron energy level is lowest in energy? 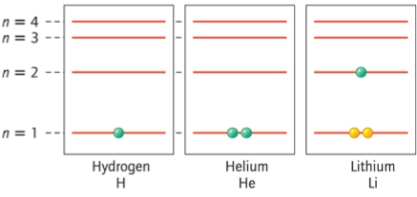
A) n = 1
B) n = 2
C) n = 3
D) n = 4
E) All electrons are equal in energy.

A) n = 1
B) n = 2
C) n = 3
D) n = 4
E) All electrons are equal in energy.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
22
Radioactive decay is the process by which
A) radioisotopes become more stable.
B) radioisotopes emit radiation.
C) radioisotopes emit high-energy particles and/or electromagnetic radiation.
D) an element of one type can change to an element of another type.
E) All of the above are correct about radioactive decay.
A) radioisotopes become more stable.
B) radioisotopes emit radiation.
C) radioisotopes emit high-energy particles and/or electromagnetic radiation.
D) an element of one type can change to an element of another type.
E) All of the above are correct about radioactive decay.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
23
The periods are the _____ of the periodic table.
A) transition metals
B) halogens
C) rows
D) columns
E) numbers
A) transition metals
B) halogens
C) rows
D) columns
E) numbers

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
24
Which pair correctly matches an element to its atomic number?
A) 9.012 - Be
B) 12.01 - C
C) 39.94 - Ar
D) 9 - F
E) 133 - Cs
A) 9.012 - Be
B) 12.01 - C
C) 39.94 - Ar
D) 9 - F
E) 133 - Cs

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
25
Most of the micronutrients are
A) transition metals.
B) metalloids.
C) nonmetals.
D) alkali earth metals.
E) noble gases.
A) transition metals.
B) metalloids.
C) nonmetals.
D) alkali earth metals.
E) noble gases.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
26
An element is a solid at room temperature and a shiny, metallic gray.However, it is a poor conductor of electricity and temperature, and it is also brittle.Which element fits this description?
A) oxygen
B) lithium
C) helium
D) antimony
E) iron
A) oxygen
B) lithium
C) helium
D) antimony
E) iron

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
27
The symbol 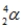 is used to represent a(n)
is used to represent a(n)
A) proton.
B) alpha particle.
C) gamma ray.
D) beta particle.
E) neutron.
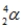 is used to represent a(n)
is used to represent a(n)A) proton.
B) alpha particle.
C) gamma ray.
D) beta particle.
E) neutron.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
28
According to the graph, what is the half-life in years of 226Ra? 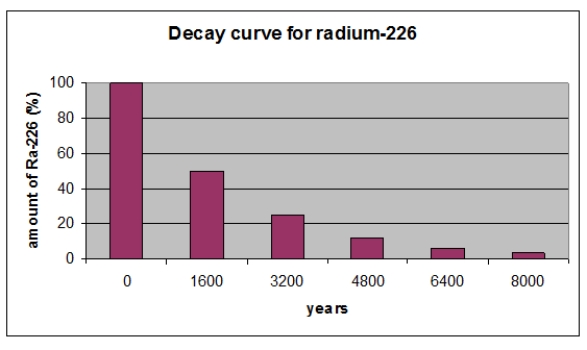
A) 8,000 years
B) 6,400 years
C) 4,800 years
D) 3,200 years
E) 1,600 years

A) 8,000 years
B) 6,400 years
C) 4,800 years
D) 3,200 years
E) 1,600 years

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
29
_____ are neutral.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
30
Electron energy levels closest to the nucleus are occupied before electron energy levels farther from the nucleus because the electron energy levels closest to the nucleus are
A) pulled toward the nucleus by gravity.
B) protected by the outer shell.
C) attracted to the nucleus for reasons unknown.
D) more stable than electrons farther from the nucleus.
E) Actually, electrons farther from the nucleus are filled first.
A) pulled toward the nucleus by gravity.
B) protected by the outer shell.
C) attracted to the nucleus for reasons unknown.
D) more stable than electrons farther from the nucleus.
E) Actually, electrons farther from the nucleus are filled first.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
31
Which pair does NOT correctly match an element symbol to its full name?
A) C - carbon
B) O - oxygen
C) H - helium
D) N - nitrogen
E) Cl - chlorine
A) C - carbon
B) O - oxygen
C) H - helium
D) N - nitrogen
E) Cl - chlorine

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
32
Why is it necessary to shield yourself from gamma radiation and beta and alpha particles but not from radio waves or microwaves?
A) Radio waves and microwaves do not have much penetrating power.
B) Radio waves and microwaves are lower in energy, so they are not ionizing.
C) Radio waves and microwaves are higher in energy, so they pass through the body without adverse effect.
D) Radio waves and microwaves are not electromagnetic radiation.
E) Gamma radiation and beta and alpha particles are not ionizing.
A) Radio waves and microwaves do not have much penetrating power.
B) Radio waves and microwaves are lower in energy, so they are not ionizing.
C) Radio waves and microwaves are higher in energy, so they pass through the body without adverse effect.
D) Radio waves and microwaves are not electromagnetic radiation.
E) Gamma radiation and beta and alpha particles are not ionizing.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
33
_____ have a mass of approximately 1 amu.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
34
Which element is depicted in this drawing of a neutral atom? 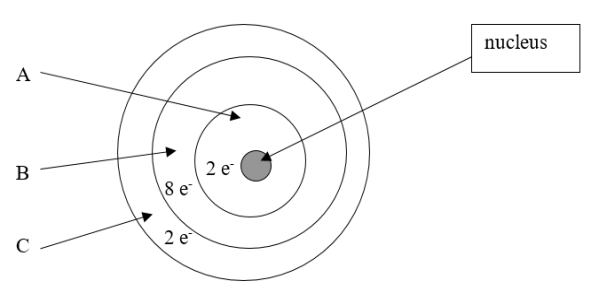
A) beryllium
B) magnesium
C) oxygen
D) neon
E) calcium

A) beryllium
B) magnesium
C) oxygen
D) neon
E) calcium

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
35
_____ are the subatomic particles that have the smallest mass.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is NOT the same for different isotopes of the same element?
A) atomic number
B) number of protons
C) number of electrons
D) charge
E) mass number
A) atomic number
B) number of protons
C) number of electrons
D) charge
E) mass number

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
37
_____ have a positive charge.
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons
A) Protons
B) Neutrons
C) Electrons
D) Protons and neutrons
E) Protons, neutrons, and electrons

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
38
What is the minimum dosage in which people are observed to die from radiation sickness? 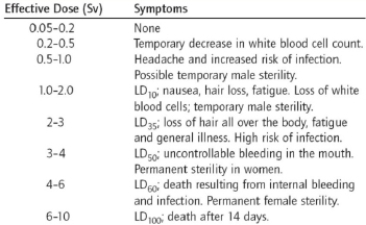
A) 0.2-0.5 Sv
B) 0.5-1.0 Sv
C) 1.0-2.0 Sv
D) 2-3 Sv
E) 3-4 Sv

A) 0.2-0.5 Sv
B) 0.5-1.0 Sv
C) 1.0-2.0 Sv
D) 2-3 Sv
E) 3-4 Sv

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
39
Iodine-131 has a half-life of 8 days.How many half-lives have passed after 24 days?
A) 1 half-life
B) 2 half-lives
C) 3 half-lives
D) 4 half-lives
E) 5 half-lives
A) 1 half-life
B) 2 half-lives
C) 3 half-lives
D) 4 half-lives
E) 5 half-lives

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
40
According to the periodic table, how many valence electrons do the elements in group 7A have?
A) 5
B) 6
C) 7
D) 8
E) It depends on the specific element.
A) 5
B) 6
C) 7
D) 8
E) It depends on the specific element.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
41
What sort of protection should be used when working with gamma emitters?
A) a lead shield
B) heavy clothing
C) a sheet of aluminum
D) a very thick slab of concrete
E) None is needed at all because they are not very penetrating.
A) a lead shield
B) heavy clothing
C) a sheet of aluminum
D) a very thick slab of concrete
E) None is needed at all because they are not very penetrating.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
42
What sort of information do the units used in this table take into account? 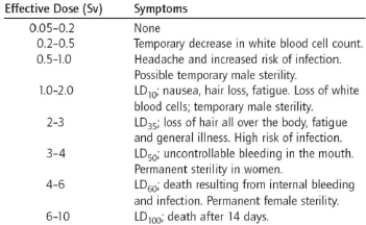
A) energy of the radiation
B) penetrating ability of the radiation
C) quality factor of the radiation
D) quantity of the radiation
E) All of the above are taken into account.

A) energy of the radiation
B) penetrating ability of the radiation
C) quality factor of the radiation
D) quantity of the radiation
E) All of the above are taken into account.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the imaging technique(s)listed below is harmless to the patient? I.x-ray
II)computed tomography
A) I only
B) II only
C) I and II
D) neither I nor II
II)computed tomography
A) I only
B) II only
C) I and II
D) neither I nor II

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
44
The product from the alpha decay of radon-222 is
A) polonium-218.
B) radium-226.
C) polonium-226.
D) radium-218.
E) lead-220.
A) polonium-218.
B) radium-226.
C) polonium-226.
D) radium-218.
E) lead-220.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
45
According to the periodic table, which element is found in period 2, group 5A?
A) nitrogen
B) vanadium
C) strontium
D) boron
E) cadmium
A) nitrogen
B) vanadium
C) strontium
D) boron
E) cadmium

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
46
Phosphorous-32 is a beta emitter with a half-life of 14.3 days.After 42 days, a 100-mg sample will have decayed to 25 mg.Which statement BEST describes where the rest of the 32P went?
A) It disappeared.
B) It reacted with air.
C) It turned into sulfur and a beta particle.
D) It turned into silicon and a beta particle.
E) It decomposed into beta particles.
A) It disappeared.
B) It reacted with air.
C) It turned into sulfur and a beta particle.
D) It turned into silicon and a beta particle.
E) It decomposed into beta particles.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
47
Which statement BEST describes why alpha particles are not frequently used in medical applications?
A) Their half-lives are too long.
B) They can do too much damage at close range.
C) They are difficult to transport.
D) They require too much shielding.
E) Their penetrating power is too great.
A) Their half-lives are too long.
B) They can do too much damage at close range.
C) They are difficult to transport.
D) They require too much shielding.
E) Their penetrating power is too great.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
48
The process in which a nucleus spontaneously breaks down by emitting radiation is known as
A) fusion.
B) fission.
C) a chain reaction.
D) reaction.
E) radioactive decay.
A) fusion.
B) fission.
C) a chain reaction.
D) reaction.
E) radioactive decay.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
49
Carbon-11 has a half-life of 20 minutes.Which of the following equations is used to calculate the amount of carbon-11 remaining after 1 hour if the starting material is a 100-mg sample?
A) 100 mg × ½
B) 100 mg/3
C) 100 mg/20
D) 100 mg/20/20/20
E) 100 mg × ½ × ½ × ½
A) 100 mg × ½
B) 100 mg/3
C) 100 mg/20
D) 100 mg/20/20/20
E) 100 mg × ½ × ½ × ½

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
50
According to the periodic table, what types of elements are in group 7A?
A) transition metals
B) noble gases
C) alkaline earth metals
D) alkali metals
E) halogens
A) transition metals
B) noble gases
C) alkaline earth metals
D) alkali metals
E) halogens

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
51
Which statement BEST interprets the statement below? The LD50 for radiation is an acute dose of 3-4 Sv.
A) Exposure to 50 mg of a radiation source will result in radiation poisoning.
B) Exposure to 50 mg of a radiation source will result in death.
C) Fifty percent of people exposed to this dose will get sick within one month of the exposure.
D) Fifty percent of people exposed to this dose will die within one month of the exposure.
E) 4 to 6 Sv is the maximum dose that 50% of people can undergo without long-term injury.
A) Exposure to 50 mg of a radiation source will result in radiation poisoning.
B) Exposure to 50 mg of a radiation source will result in death.
C) Fifty percent of people exposed to this dose will get sick within one month of the exposure.
D) Fifty percent of people exposed to this dose will die within one month of the exposure.
E) 4 to 6 Sv is the maximum dose that 50% of people can undergo without long-term injury.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
52
_____ is an important component of the immune system as well as required by many enzymes.
A) Iodine
B) Fluorine
C) Zinc
D) Iron
E) Oxygen
A) Iodine
B) Fluorine
C) Zinc
D) Iron
E) Oxygen

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following colors has the lowest energy?
A) red
B) orange
C) yellow
D) green
E) blue
A) red
B) orange
C) yellow
D) green
E) blue

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
54
How many electrons are in a neutral atom of element X? 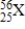
A) 25
B) 56
C) 81
D) 31
E) None of the above values is the number of electrons.
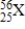
A) 25
B) 56
C) 81
D) 31
E) None of the above values is the number of electrons.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
55
According to the periodic table, which of the following sets of terms accurately describes potassium?
Nonmetal Halogen Alkali metal Group 2A
I II III IV
A) I only
B) III only
C) I and II
D) II and IV
E) III and IV
Nonmetal Halogen Alkali metal Group 2A
I II III IV
A) I only
B) III only
C) I and II
D) II and IV
E) III and IV

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
56
In a balanced nuclear reaction, which of the following is consistent with the release of a gamma particle?
A) The identity of the atom does not change.
B) All radioactive decay releases gamma radiation.
C) The mass number decreases by 4.
D) The atomic number increases by 1.
E) The atomic number decreases by 1.
A) The identity of the atom does not change.
B) All radioactive decay releases gamma radiation.
C) The mass number decreases by 4.
D) The atomic number increases by 1.
E) The atomic number decreases by 1.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
57
Which element would you expect to be the largest?
A) fluorine
B) chlorine
C) argon
D) calcium
E) hydrogen
A) fluorine
B) chlorine
C) argon
D) calcium
E) hydrogen

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
58
Micro- and macronutrients are
A) equally distributed throughout the body.
B) all metals and metalloids.
C) obtained through the diet.
D) only found in the first three periods of the periodic table.
E) all required in quantities of more than 100 mg per day.
A) equally distributed throughout the body.
B) all metals and metalloids.
C) obtained through the diet.
D) only found in the first three periods of the periodic table.
E) all required in quantities of more than 100 mg per day.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following indicates that an alpha particle has been released during radioactive decay of an atom?
A) The identity of the atom does not change.
B) The mass number of the atom decreases by 4.
C) The atomic number of the atom increases by 1
D) The atomic number of the atom decreases by 1.
E) The atomic number of the atom decreases by 4.
A) The identity of the atom does not change.
B) The mass number of the atom decreases by 4.
C) The atomic number of the atom increases by 1
D) The atomic number of the atom decreases by 1.
E) The atomic number of the atom decreases by 4.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
60
_____ make up the majority of compounds found in living organisms.
A) Building-block elements
B) Macronutrients
C) Micronutrients
D) Metals
E) Metalloids
A) Building-block elements
B) Macronutrients
C) Micronutrients
D) Metals
E) Metalloids

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
61
Which statement about the model of the atom is TRUE?
A) The nucleus is much less dense than the surrounding electrons.
B) Electrons orbit the nucleus like planets around the Sun.
C) This is the first model of the atom.
D) The atom is mostly empty space.
E) The model of the atom was developed by looking directly at an atom.
A) The nucleus is much less dense than the surrounding electrons.
B) Electrons orbit the nucleus like planets around the Sun.
C) This is the first model of the atom.
D) The atom is mostly empty space.
E) The model of the atom was developed by looking directly at an atom.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
62
Effective dose measurements take into account the ______ of a type of radiation.
A) energy
B) penetrating ability
C) biological effect
D) quantity
E) All of the above are taken into account for the effective dose measurement.
A) energy
B) penetrating ability
C) biological effect
D) quantity
E) All of the above are taken into account for the effective dose measurement.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
63
According to the periodic table, what types of elements are in group 8A?
A) transition metals
B) noble gases
C) alkaline earth metals
D) alkali metals
E) halogens
A) transition metals
B) noble gases
C) alkaline earth metals
D) alkali metals
E) halogens

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
64
What is a common symptom of iodine deficiency?
A) weak bones and teeth
B) enlarged thyroid
C) anemia
D) slow wound healing
E) All of these are common symptoms.
A) weak bones and teeth
B) enlarged thyroid
C) anemia
D) slow wound healing
E) All of these are common symptoms.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
65
According to the periodic table, how many valence electrons do the elements in the third row have?
A) 3
B) 4
C) 5
D) 8
E) It depends on the specific element.
A) 3
B) 4
C) 5
D) 8
E) It depends on the specific element.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
66
In a balanced nuclear reaction, which statement is consistent with the release of a beta particle?
A) The identity of the atom does not change.
B) All radioactive decay releases a beta particle.
C) The mass number decreases by 4.
D) The atomic number increases by 1.
E) The atomic number decreases by 1.
A) The identity of the atom does not change.
B) All radioactive decay releases a beta particle.
C) The mass number decreases by 4.
D) The atomic number increases by 1.
E) The atomic number decreases by 1.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
67
The nucleus is composed of _____.
A) protons
B) neutrons
C) electrons
D) protons and neutrons
E) protons, neutrons, and electrons
A) protons
B) neutrons
C) electrons
D) protons and neutrons
E) protons, neutrons, and electrons

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
68
Radioactive isotopes are
A) very stable isotopes.
B) highly chemically reactive.
C) unstable isotopes.
D) charged species.
E) unusually nonreactive.
A) very stable isotopes.
B) highly chemically reactive.
C) unstable isotopes.
D) charged species.
E) unusually nonreactive.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
69
The average atomic mass of zirconium is
Isotope
Natural abundance
A
90Zr
52%
B
91Zr
11%
C
92Zr
17%
D
94Zr
17%
E
96Zr
3%
A) less than 90 because the atomic mass only depends on the number of protons in the atom.
B) 90 because 90Zn has the highest natural abundance.
C) greater than 90 but less than 96 because the atomic mass takes into account the abundance of all naturally occurring isotopes.
D) 96 because the atomic mass is the mass of the highest naturally occurring isotope.
E) greater than 96 because the atomic mass is the sum of masses of the naturally occurring isotopes.
Isotope
Natural abundance
A
90Zr
52%
B
91Zr
11%
C
92Zr
17%
D
94Zr
17%
E
96Zr
3%
A) less than 90 because the atomic mass only depends on the number of protons in the atom.
B) 90 because 90Zn has the highest natural abundance.
C) greater than 90 but less than 96 because the atomic mass takes into account the abundance of all naturally occurring isotopes.
D) 96 because the atomic mass is the mass of the highest naturally occurring isotope.
E) greater than 96 because the atomic mass is the sum of masses of the naturally occurring isotopes.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
70
What is the most penetrating electromagnetic radiation?
A) radio waves
B) microwaves
C) gamma rays
D) visible light
E) ultraviolet rays
A) radio waves
B) microwaves
C) gamma rays
D) visible light
E) ultraviolet rays

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
71
How does computed tomography (CT)differ from standard x-ray imaging?
A) CT scans involve software as well as x-ray imaging.
B) CT scans use an array of detectors.
C) CT scans are 3D; X-rays are 2D.
D) All of the above list how CT scans differ from x-ray imaging.
A) CT scans involve software as well as x-ray imaging.
B) CT scans use an array of detectors.
C) CT scans are 3D; X-rays are 2D.
D) All of the above list how CT scans differ from x-ray imaging.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
72
Adding _____ to drinking water is a common practice in many cities, meant to strengthen tooth enamel and decrease dental cavities.
A) iodine
B) fluorine
C) zinc
D) iron
E) oxygen
A) iodine
B) fluorine
C) zinc
D) iron
E) oxygen

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
73
_____ is an important component of hemoglobin.Without this protein, tissues become starved of oxygen, and fatigue and shortness of breath results.
A) Iodine
B) Fluorine
C) Zinc
D) Iron
E) Oxygen
A) Iodine
B) Fluorine
C) Zinc
D) Iron
E) Oxygen

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following describes a benefit of using Sieverts instead of grays to measure the quantity of radiation that a patient has received?
A) Sieverts measure the biological effect of radiation, not just quantity.
B) Grays measure the biological effect of radiation, not just quantity.
C) Sieverts measure the amount of energy absorbed, not just quantity.
D) Grays measure the amount of energy absorbed, not just quantity.
E) There is no benefit.
A) Sieverts measure the biological effect of radiation, not just quantity.
B) Grays measure the biological effect of radiation, not just quantity.
C) Sieverts measure the amount of energy absorbed, not just quantity.
D) Grays measure the amount of energy absorbed, not just quantity.
E) There is no benefit.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
75
An atom in a metastable state is
A) unusually low in energy.
B) unusually stable.
C) very unreactive.
D) high in energy.
E) emittable.
A) unusually low in energy.
B) unusually stable.
C) very unreactive.
D) high in energy.
E) emittable.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
76
Isotopes are elements with the same number of
A) electrons but different numbers of protons.
B) protons but different numbers of electrons.
C) electrons but different numbers of neutrons.
D) protons but different numbers of neutrons.
E) neutrons but different numbers of protons.
A) electrons but different numbers of protons.
B) protons but different numbers of electrons.
C) electrons but different numbers of neutrons.
D) protons but different numbers of neutrons.
E) neutrons but different numbers of protons.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
77
The radioisotope undergoing decay is often called the
A) reactant.
B) product.
C) parent nuclide.
D) decayer.
E) daughter nuclide.
A) reactant.
B) product.
C) parent nuclide.
D) decayer.
E) daughter nuclide.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
78
According to the periodic table, what types of elements are in group 2A?
A) transition metals
B) noble gases
C) alkaline earth metals
D) alkali metals
E) halogens
A) transition metals
B) noble gases
C) alkaline earth metals
D) alkali metals
E) halogens

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
79
The time that it takes a macroscopic sample of a radioisotope to decay to one-half its original activity is known as the
A) reaction rate.
B) kinetics.
C) half-life.
D) lifetime.
E) decay rate.
A) reaction rate.
B) kinetics.
C) half-life.
D) lifetime.
E) decay rate.

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following elements exists as an isotope with a mass number of 35 and an atomic number of 17?
A) chlorine
B) bromine
C) argon
D) tellurium
E) sulfur-35
A) chlorine
B) bromine
C) argon
D) tellurium
E) sulfur-35

Unlock Deck
Unlock for access to all 107 flashcards in this deck.
Unlock Deck
k this deck


