Exam 2: Atomic Structure and Radioisotopes
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Which pair correctly matches an element to its atomic number?
Free
(Multiple Choice)
4.9/5  (43)
(43)
Correct Answer:
D
In which of the following nuclear reactions is only gamma radiation released?
Free
(Multiple Choice)
4.8/5  (40)
(40)
Correct Answer:
A
Which statement BEST describes why alpha particles are not frequently used in medical applications?
Free
(Multiple Choice)
4.8/5  (43)
(43)
Correct Answer:
B
Which of the method(s)of imaging listed below uses x-rays? I.x-ray
II)computed tomography
(Multiple Choice)
4.8/5  (35)
(35)
Carbon-11 has a half-life of 20 minutes.Which of the following equations is used to calculate the amount of carbon-11 remaining after 1 hour if the starting material is a 100-mg sample?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following indicates that an alpha particle has been released during radioactive decay of an atom?
(Multiple Choice)
4.8/5  (37)
(37)
_____ determine the physical and chemical characteristics of an atom.
(Multiple Choice)
4.8/5  (29)
(29)
Which pair does NOT correctly match an element symbol to its full name?
(Multiple Choice)
4.8/5  (25)
(25)
According to the current model of the atom, the part of the diagram labeled A is made up of 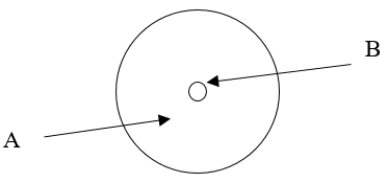
(Multiple Choice)
4.9/5  (31)
(31)
Which of the following elements exists as an isotope with a mass number of 35 and an atomic number of 17?
(Multiple Choice)
4.7/5  (36)
(36)
Adding _____ to drinking water is a common practice in many cities, meant to strengthen tooth enamel and decrease dental cavities.
(Multiple Choice)
4.9/5  (31)
(31)
The number of protons is equal to the _____ in a neutral atom.
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following terms is NOT a characteristic of a metal?
(Multiple Choice)
4.8/5  (33)
(33)
According to the periodic table, the atomic mass of potassium (K)is ______.
(Multiple Choice)
4.8/5  (40)
(40)
Showing 1 - 20 of 107
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)