Deck 1: Matter and Energy: The Origin of the Universe
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 1: Matter and Energy: The Origin of the Universe
1
Identify the incorrect statement(s). A pure substance can be _____
I. an element or a compound.
II. heterogeneous or homogeneous.
III. a solution.
A) Only I is incorrect.
B) Only II is incorrect.
C) Only III is incorrect.
D) Both I and II are incorrect.
E) Both II and III are incorrect.
I. an element or a compound.
II. heterogeneous or homogeneous.
III. a solution.
A) Only I is incorrect.
B) Only II is incorrect.
C) Only III is incorrect.
D) Both I and II are incorrect.
E) Both II and III are incorrect.
Both II and III are incorrect.
2
Which one of the following is not a physical process or change?
A) natural gas burning
B) water boiling
C) ice melting
D) iodine vaporizing
E) alcohol evaporating
A) natural gas burning
B) water boiling
C) ice melting
D) iodine vaporizing
E) alcohol evaporating
natural gas burning
3
Table sugar (sucrose) with the formula C12H22O11 is _____
I. an element.
II. a compound.
III. a mixture.
A) Only I
B) Only II
C) Only III
D) Both I and III
E) Both II and III
I. an element.
II. a compound.
III. a mixture.
A) Only I
B) Only II
C) Only III
D) Both I and III
E) Both II and III
Only II
4
Which of the following is not a pure substance?
A) air
B) nitrogen gas
C) oxygen gas
D) argon gas
E) table salt (sodium chloride)
A) air
B) nitrogen gas
C) oxygen gas
D) argon gas
E) table salt (sodium chloride)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
Which one of the following is not a correct statement?
A) Vodka is a solution.
B) Water (H2O) is a compound.
C) Sodium chloride (table salt) is a compound.
D) Silver is an element.
E) Sugar dissolved in water is a heterogeneous mixture.
A) Vodka is a solution.
B) Water (H2O) is a compound.
C) Sodium chloride (table salt) is a compound.
D) Silver is an element.
E) Sugar dissolved in water is a heterogeneous mixture.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following is not classified correctly?
A) Distilled water is a compound.
B) Gold is an element.
C) Air is a solution.
D) Table salt (sodium chloride) is a mixture.
E) Salad dressing is a suspension.
A) Distilled water is a compound.
B) Gold is an element.
C) Air is a solution.
D) Table salt (sodium chloride) is a mixture.
E) Salad dressing is a suspension.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is a homogeneous mixture?
A) filtered water
B) chicken noodle soup
C) clouds
D) trail mix snack
E) fruit salad
A) filtered water
B) chicken noodle soup
C) clouds
D) trail mix snack
E) fruit salad

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
Which one of the following is not a chemical reaction?
A) dynamite exploding
B) iron rusting
C) wood burning
D) water turning to steam
E) eggs cooking
A) dynamite exploding
B) iron rusting
C) wood burning
D) water turning to steam
E) eggs cooking

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
An element __________
A) can be separated into its components by physical methods.
B) may have different chemical properties depending on its source.
C) can not be separated into simpler substances by chemical methods.
D) can also be a compound.
E) exists only as atoms and not as molecules.
A) can be separated into its components by physical methods.
B) may have different chemical properties depending on its source.
C) can not be separated into simpler substances by chemical methods.
D) can also be a compound.
E) exists only as atoms and not as molecules.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
Identify the incorrect statement(s). A solution _____
I. can be a solid, liquid, or gas.
II. can be heterogeneous or homogeneous.
III. is a homogeneous mixture.
A) Only I is incorrect.
B) Only II is incorrect.
C) Only III is incorrect.
D) Both I and II are incorrect.
E) Both I and III are incorrect.
I. can be a solid, liquid, or gas.
II. can be heterogeneous or homogeneous.
III. is a homogeneous mixture.
A) Only I is incorrect.
B) Only II is incorrect.
C) Only III is incorrect.
D) Both I and II are incorrect.
E) Both I and III are incorrect.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following statements is not correct?
A) Helium is an element.
B) Table salt (sodium chloride) is a compound.
C) Water is a pure substance.
D) Air is a solution.
E) Elements occur only in the form of individual atoms.
A) Helium is an element.
B) Table salt (sodium chloride) is a compound.
C) Water is a pure substance.
D) Air is a solution.
E) Elements occur only in the form of individual atoms.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is an element?
A) Cl2
B) H2O
C) NaCl
D) MgO
E) HCl
A) Cl2
B) H2O
C) NaCl
D) MgO
E) HCl

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following statements is not correct?
A) A compound has a specific constant composition.
B) The composition of a mixture can vary.
C) A compound has specific constant properties.
D) The properties of a mixture can vary.
E) Mixtures can not be homogeneous.
A) A compound has a specific constant composition.
B) The composition of a mixture can vary.
C) A compound has specific constant properties.
D) The properties of a mixture can vary.
E) Mixtures can not be homogeneous.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
Which one of the following statements is not correct?
A) Sodium and chlorine are elements.
B) Sodium chloride (table salt) is a compound.
C) Sodium chloride is a pure substance.
D) Sodium chloride is a heterogeneous mixture.
E) Sodium chloride added to water forms a solution.
A) Sodium and chlorine are elements.
B) Sodium chloride (table salt) is a compound.
C) Sodium chloride is a pure substance.
D) Sodium chloride is a heterogeneous mixture.
E) Sodium chloride added to water forms a solution.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of the following statements is not correct?
A) Dry ice subliming is a physical change.
B) Methanol burning is a chemical reaction.
C) Sugar dissolving in water is a physical change.
D) Bleaching your hair is a chemical change (reaction), even though it changes your physical appearance.
E) Liquid water turning into steam is a chemical reaction.
A) Dry ice subliming is a physical change.
B) Methanol burning is a chemical reaction.
C) Sugar dissolving in water is a physical change.
D) Bleaching your hair is a chemical change (reaction), even though it changes your physical appearance.
E) Liquid water turning into steam is a chemical reaction.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
Air is an example of __________
A) an element.
B) a compound.
C) a pure substance.
D) a heterogeneous mixture.
E) a homogeneous mixture.
A) an element.
B) a compound.
C) a pure substance.
D) a heterogeneous mixture.
E) a homogeneous mixture.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following is a pure substance?
A) mineral water
B) blood
C) brass (an alloy of copper and zinc)
D) sucrose (table sugar)
E) beer
A) mineral water
B) blood
C) brass (an alloy of copper and zinc)
D) sucrose (table sugar)
E) beer

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
A pure substance __________
A) can not be separated into simpler substances by physical means.
B) can have a composition that varies from sample to sample.
C) must be an element.
D) has different chemical and physical properties depending on its source.
E) must be a compound.
A) can not be separated into simpler substances by physical means.
B) can have a composition that varies from sample to sample.
C) must be an element.
D) has different chemical and physical properties depending on its source.
E) must be a compound.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following is a mixture?
A) an aqueous solution of sugar
B) pure water
C) nitrogen gas
D) copper metal
E) table salt (sodium chloride)
A) an aqueous solution of sugar
B) pure water
C) nitrogen gas
D) copper metal
E) table salt (sodium chloride)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following is a heterogeneous mixture?
A) air
B) sugar dissolved in water
C) muddy river water
D) brass (an alloy of copper and zinc)
E) table salt (sodium chloride)
A) air
B) sugar dissolved in water
C) muddy river water
D) brass (an alloy of copper and zinc)
E) table salt (sodium chloride)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following processes is a chemical reaction?
A) distillation
B) combustion
C) filtration
D) condensation
E) sublimation
A) distillation
B) combustion
C) filtration
D) condensation
E) sublimation

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
In the movie The Italian Job, thieves steal gold bullion. One plan was to carry the ingots of gold off in suitcases. If each suitcase were 19 inches *14 inches * 10 inches, approximately how much would each suitcase weigh when filled with gold? The volume of each suitcase is 4.4 * 104 mL, the molar mass of gold is 197 g/mol, and the density of gold is 19.3 g/mL.
A) 2,300 g
B) 850 kg
C) 4,300 g
D) 167 mg
E) 550 kg
A) 2,300 g
B) 850 kg
C) 4,300 g
D) 167 mg
E) 550 kg

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
Distillation may be used to separate components in a mixture based on differences in __________
A) solubilities.
B) boiling points.
C) melting points.
D) masses.
E) color.
A) solubilities.
B) boiling points.
C) melting points.
D) masses.
E) color.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
Which statement A-D about the reaction of methane with oxygen, which is called combustion and is represented by the reaction equation below, is not correct? The reaction products are carbon dioxide and water.
CH4 + 2O2 CO2 + 2H2O
A) One molecule of methane combines with two molecules of oxygen.
B) The products are one molecule of carbon dioxide and two molecules of water.
C) The equation is balanced because the number of atoms of each element does not change.
D) Four atoms of hydrogen combine with four atoms of oxygen to produce water.
E) Statements A-D all are correct.
CH4 + 2O2 CO2 + 2H2O
A) One molecule of methane combines with two molecules of oxygen.
B) The products are one molecule of carbon dioxide and two molecules of water.
C) The equation is balanced because the number of atoms of each element does not change.
D) Four atoms of hydrogen combine with four atoms of oxygen to produce water.
E) Statements A-D all are correct.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
An example of a chemical property of formaldehyde (CH2O) is __________
A) it is flammable.
B) it has a density of 1.09 g/mL.
C) it is colorless.
D) it dissolves in water.
E) it is a gas at room temperature.
A) it is flammable.
B) it has a density of 1.09 g/mL.
C) it is colorless.
D) it dissolves in water.
E) it is a gas at room temperature.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following is a chemical property?
A) Hydrogen is flammable.
B) Hydrogen is a gas.
C) Hydrogen gas has mass.
D) The boiling point of hydrogen is 20 K.
E) Hydrogen gas exerts pressure on the walls of a container.
A) Hydrogen is flammable.
B) Hydrogen is a gas.
C) Hydrogen gas has mass.
D) The boiling point of hydrogen is 20 K.
E) Hydrogen gas exerts pressure on the walls of a container.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following represents a physical property of water?
A) Water boils at 100°C.
B) An electrical current decomposes water into hydrogen gas and oxygen gas.
C) Water reacts with iron metal and oxygen to form rust.
D) Water reacts with carbon monoxide to form carbon dioxide and hydrogen gas.
E) Water is used in photosynthesis.
A) Water boils at 100°C.
B) An electrical current decomposes water into hydrogen gas and oxygen gas.
C) Water reacts with iron metal and oxygen to form rust.
D) Water reacts with carbon monoxide to form carbon dioxide and hydrogen gas.
E) Water is used in photosynthesis.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
Which one of the following is not a physical property?
A) flammability
B) electrical conductivity
C) color
D) density
E) boiling point
A) flammability
B) electrical conductivity
C) color
D) density
E) boiling point

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
In the movie The Italian Job, thieves steal gold bullion. One plan was to carry the ingots of gold off in suitcases. If each suitcase were 24 inches *18 inches*12 inches, approximately how much would each suitcase weigh when filled with gold? The molar mass of gold is 197 g/mol, the density of gold is 19.3 g/cm3, 1 in = 2.54 cm, and 1 kg = 2.20 lb.
A) 3,600 lb
B) 560 lb
C) 2,200 lb
D) 280 lb
E) 1,800 lb
A) 3,600 lb
B) 560 lb
C) 2,200 lb
D) 280 lb
E) 1,800 lb

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
Filtration can be used to separate components in a mixture based on differences in _____
A) solubility.
B) boiling point.
C) melting point.
D) particle size.
E) color.
A) solubility.
B) boiling point.
C) melting point.
D) particle size.
E) color.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
The density of an object that weighs 10.0 g and occupies a volume of 2.5 cm3 is __________
A) 4.0 g/cm3.
B) 4.0 cm3/g.
C) 0.25 g/cm3.
D) 0.25 cm3/g.
E) 25 g/cm3.
A) 4.0 g/cm3.
B) 4.0 cm3/g.
C) 0.25 g/cm3.
D) 0.25 cm3/g.
E) 25 g/cm3.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
Molecules are represented in various ways. Which statement A-D about molecular representations is not correct.
A) A molecular or chemical formula identifies the elements and the number of atoms of each that comprise a molecule of a compound.
B) A structural formula shows how the atoms are bonded together but does not necessarily indicate the bond angles or three-dimensional shape of the molecule.
C) A ball-and-stick model shows bond angles and the three-dimensional shape of a molecule.
D) A space-filling model best represents the size of the atoms and distribution of electrons in a molecule.
E) Statements A-D all are correct.
A) A molecular or chemical formula identifies the elements and the number of atoms of each that comprise a molecule of a compound.
B) A structural formula shows how the atoms are bonded together but does not necessarily indicate the bond angles or three-dimensional shape of the molecule.
C) A ball-and-stick model shows bond angles and the three-dimensional shape of a molecule.
D) A space-filling model best represents the size of the atoms and distribution of electrons in a molecule.
E) Statements A-D all are correct.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
The law of constant composition states that _________
A) compounds such as NO2 and SO2 have identical chemical properties.
B) the elements forming a particular compound always combine in the same proportions.
C) nitrogen and oxygen can combine to form NO or NO2.
D) compounds such as NO and NO2 have identical chemical properties.
E) only one compound can be produced when two elements combine.
A) compounds such as NO2 and SO2 have identical chemical properties.
B) the elements forming a particular compound always combine in the same proportions.
C) nitrogen and oxygen can combine to form NO or NO2.
D) compounds such as NO and NO2 have identical chemical properties.
E) only one compound can be produced when two elements combine.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
Jupiter's mass is estimated to be 1.90 * 1027 kg, and it has a diameter of 142,984 km. Assuming that Jupiter is spherical, estimate its density (the volume of a sphere is 4 r3/3).
A) 0.620 g/cm3
B) 1.61 g/cm3
C) 1.24 g/cm3
D) 0.00124 g/cm3
E) 1240 g/cm3
A) 0.620 g/cm3
B) 1.61 g/cm3
C) 1.24 g/cm3
D) 0.00124 g/cm3
E) 1240 g/cm3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following represents a chemical property of copper metal?
A) Copper metal conducts heat.
B) Copper metal reacts with nitric acid to produce copper(II) nitrate.
C) Copper metal melts at 1085°C.
D) Copper metal conducts electricity.
E) Copper metal has an orange color.
A) Copper metal conducts heat.
B) Copper metal reacts with nitric acid to produce copper(II) nitrate.
C) Copper metal melts at 1085°C.
D) Copper metal conducts electricity.
E) Copper metal has an orange color.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
The density of iron is 7.9 g/cm3. What is the volume of a 4.5 kg iron block?
A) 570 cm3
B) 0.570 cm3
C) 3.56* 104 cm3
D) 35.6 cm3
E) 1.76 cm3
A) 570 cm3
B) 0.570 cm3
C) 3.56* 104 cm3
D) 35.6 cm3
E) 1.76 cm3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following mixtures can be separated by filtration?
A) sugar dissolved in coffee
B) sand and water
C) gasoline
D) alcohol dissolved in water
E) air
A) sugar dissolved in coffee
B) sand and water
C) gasoline
D) alcohol dissolved in water
E) air

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
A structural formula __________
A) always shows correct bond distances and angles in a molecule.
B) is the same as a chemical formula.
C) shows how the molecule can be synthesized.
D) shows how atoms are connected in a chemical species.
E) is the same as a molecular formula.
A) always shows correct bond distances and angles in a molecule.
B) is the same as a chemical formula.
C) shows how the molecule can be synthesized.
D) shows how atoms are connected in a chemical species.
E) is the same as a molecular formula.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
If you had equal masses of each of the following substances, which would occupy the greatest volume?
A) ice (d = 0.917 g/mL)
B) water (d = 0.997 g/mL)
C) beeswax (d = 0.960 g/mL)
D) cocoa butter (d = 0.910 g/mL)
E) aluminum (d = 2.70 g/mL)
A) ice (d = 0.917 g/mL)
B) water (d = 0.997 g/mL)
C) beeswax (d = 0.960 g/mL)
D) cocoa butter (d = 0.910 g/mL)
E) aluminum (d = 2.70 g/mL)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
How thick is a square piece of aluminum foil that measures 5 cm on each side and has a mass of
675 mg? The density of aluminum is 2.70 g/cm3.
A) 1.0 mm
B) 0.1 mm
C) 0.01 mm
D) 10 m
E) 1.0 m
675 mg? The density of aluminum is 2.70 g/cm3.
A) 1.0 mm
B) 0.1 mm
C) 0.01 mm
D) 10 m
E) 1.0 m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
A solid forming a liquid is called ___________.
A) sublimation
B) deposition
C) melting
D) freezing
E) vaporization
A) sublimation
B) deposition
C) melting
D) freezing
E) vaporization

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
The symbol and name corresponding to the factor 10-9 is
A) f, femto
B) p, pico
C) n, nano
D) ( , micro)
E) m, milli
A) f, femto
B) p, pico
C) n, nano
D) ( , micro)
E) m, milli

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is the most massive?
A) 2.5 kg of oxygen gas
B) 0.25 kg of iron
C) 2.5 g of sodium chloride (table salt)
D) 250 g of helium gas
E) 250 mg of aluminum
A) 2.5 kg of oxygen gas
B) 0.25 kg of iron
C) 2.5 g of sodium chloride (table salt)
D) 250 g of helium gas
E) 250 mg of aluminum

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
Extensive properties are __________
A) physical properties and not chemical properties.
B) identical for all substances.
C) independent of the volume of substance present.
D) dependent on the amount of substance.
E) dependent on factors external to the substance itself.
A) physical properties and not chemical properties.
B) identical for all substances.
C) independent of the volume of substance present.
D) dependent on the amount of substance.
E) dependent on factors external to the substance itself.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
A hypothesis is __________
A) an explanation that cannot be validated.
B) a scientific theory used to explain observations.
C) an explanation of observed processes that needs to be tested.
D) the entire process through which scientific phenomena are explained.
E) one side of a right triangle.
A) an explanation that cannot be validated.
B) a scientific theory used to explain observations.
C) an explanation of observed processes that needs to be tested.
D) the entire process through which scientific phenomena are explained.
E) one side of a right triangle.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
When you place a piece of dry ice (solid carbon dioxide) on a plate at room temperature, you notice that no liquid forms, unlike ice that melts to form liquid water. This is because dry ice __________
A) as a liquid quickly evaporates.
B) undergoes deposition instead of melting.
C) sublimes instead of melting.
D) does not exist in the liquid form at room temperature and pressure.
E) contains no water.
A) as a liquid quickly evaporates.
B) undergoes deposition instead of melting.
C) sublimes instead of melting.
D) does not exist in the liquid form at room temperature and pressure.
E) contains no water.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is a derived SI unit?
A) m
B) kg
C) cm3
D) m3
E) lb
A) m
B) kg
C) cm3
D) m3
E) lb

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
The symbol and name corresponding to the factor 10-6 is
A) f, femto
B) p, pico
C) n, nano
D) ( , micro)
E) m, milli
A) f, femto
B) p, pico
C) n, nano
D) ( , micro)
E) m, milli

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
Which represents an intensive property?
A) Hydrogen gas has mass.
B) Hydrogen gas has a given density.
C) A balloon filled with hydrogen gas has a given volume.
D) Hydrogen releases a given amount of energy when it reacts with oxygen.
E) Hydrogen gas in a steel tank exerts a given pressure.
A) Hydrogen gas has mass.
B) Hydrogen gas has a given density.
C) A balloon filled with hydrogen gas has a given volume.
D) Hydrogen releases a given amount of energy when it reacts with oxygen.
E) Hydrogen gas in a steel tank exerts a given pressure.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
John Dalton postulated that all matter is composed of small particles called atoms. For this proposition to be considered a valid scientific theory, __________
A) it must be supported by experimental evidence and testing.
B) it must be impossible to prove wrong by experiment.
C) all possible experiments must never find an exception to it.
D) some, but only a few, experiments may find exceptions to it.
E) it must be voted on by the scientific community and accepted by all.
A) it must be supported by experimental evidence and testing.
B) it must be impossible to prove wrong by experiment.
C) all possible experiments must never find an exception to it.
D) some, but only a few, experiments may find exceptions to it.
E) it must be voted on by the scientific community and accepted by all.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
The bubbles that form in water after it has been boiling for some time are __________
A) empty space.
B) H2(g) and O2(g) gases.
C) the vapor phase of water, H2O(g).
D) filled with air.
E) superhot water, H2O(l).
A) empty space.
B) H2(g) and O2(g) gases.
C) the vapor phase of water, H2O(g).
D) filled with air.
E) superhot water, H2O(l).

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is the SI base unit for mass?
A) g
B) kg
C) mg
D) lb
E) m
A) g
B) kg
C) mg
D) lb
E) m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
A liquid forming a gas is called ___________.
A) sublimation
B) deposition
C) melting
D) freezing
E) vaporization
A) sublimation
B) deposition
C) melting
D) freezing
E) vaporization

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
A solid directly forming a vapor or gas is called ___________.
A) sublimation
B) deposition
C) melting
D) freezing
E) vaporization
A) sublimation
B) deposition
C) melting
D) freezing
E) vaporization

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
A gas forming a liquid is called ___________.
A) condensation
B) deposition
C) melting
D) freezing
E) vaporization
A) condensation
B) deposition
C) melting
D) freezing
E) vaporization

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
Which statement correctly describes the properties of a gas?
A) A gas does not occupy the entire volume of the container and is not highly compressible.
B) A gas occupies the entire volume of the container and is highly compressible.
C) A gas is highly ordered, and the molecules do not move about in the container.
D) A gas has a definite volume and shape.
E) A gas takes the shape of the container but is not highly compressible.
A) A gas does not occupy the entire volume of the container and is not highly compressible.
B) A gas occupies the entire volume of the container and is highly compressible.
C) A gas is highly ordered, and the molecules do not move about in the container.
D) A gas has a definite volume and shape.
E) A gas takes the shape of the container but is not highly compressible.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
A liquid forming a solid is called ___________.
A) sublimation
B) deposition
C) melting
D) freezing
E) vaporization
A) sublimation
B) deposition
C) melting
D) freezing
E) vaporization

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
Deposition is the process in which a __________ is converted into a __________.
A) liquid; solid
B) gas; liquid
C) gas; solid
D) liquid; gas
E) solid; liquid
A) liquid; solid
B) gas; liquid
C) gas; solid
D) liquid; gas
E) solid; liquid

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
A vapor or gas forming a solid is called ___________.
A) sublimation
B) deposition
C) melting
D) freezing
E) vaporization
A) sublimation
B) deposition
C) melting
D) freezing
E) vaporization

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is not a base SI unit?
A) cm
B) m
C) kg
D) sec
E) mol
A) cm
B) m
C) kg
D) sec
E) mol

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
The following measurements of the mass of an aspirin tablet were made by different students in a lab. Which set is the most precise?
A) 1.513 g, 1.503 g, 1.522 g
B) 1.513 g, 1.511 g, 1.450 g
C) 1.513 g, 1.459 g, 1.533 g
D) 1.513 g, 1.517 g, 1.512 g
E) 1.513 g, 1.510 g, 1.523 g
A) 1.513 g, 1.503 g, 1.522 g
B) 1.513 g, 1.511 g, 1.450 g
C) 1.513 g, 1.459 g, 1.533 g
D) 1.513 g, 1.517 g, 1.512 g
E) 1.513 g, 1.510 g, 1.523 g

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
Given the following figure, which of the measurements listed is the best estimate of the length of the aluminum rod? 
A) 1.8 cm
B) 1.81 cm
C) 1.810 cm
D) 1.9 cm
E) 2 cm

A) 1.8 cm
B) 1.81 cm
C) 1.810 cm
D) 1.9 cm
E) 2 cm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
You measure and find that an object has a mass of 25.15 g and a volume of 9.3 cm3. What would you report as the density of the object?
A) 2.704 g/cm3
B) 2.70 g/cm3
C) 2.7 g/cm3
D) 3 g/cm3
E) 2.704301075 g/cm3
A) 2.704 g/cm3
B) 2.70 g/cm3
C) 2.7 g/cm3
D) 3 g/cm3
E) 2.704301075 g/cm3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
Indicate which of the following common laboratory devices will deliver 25 mL of a solution with the greatest precision.
A) a 50 mL Erlenmeyer flask (without volume divisions)
B) a 50 mL beaker (with volume divisions every 10 mL)
C) a 50 mL graduated cylinder (with volume divisions every 2 mL)
D) a 25 mL Erlenmeyer flask (without volume divisions)
E) a 25 mL volumetric pipette (with a to-deliver error of 0.01 mL at 25°C)
A) a 50 mL Erlenmeyer flask (without volume divisions)
B) a 50 mL beaker (with volume divisions every 10 mL)
C) a 50 mL graduated cylinder (with volume divisions every 2 mL)
D) a 25 mL Erlenmeyer flask (without volume divisions)
E) a 25 mL volumetric pipette (with a to-deliver error of 0.01 mL at 25°C)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
The following measurements of the mass of an aspirin tablet were made by different students in a lab. Each set gives the results of 3 measurements followed by the average. Which set is the most precise? All values are in grams. The standard value reported by an analytical laboratory was 1.501 g.
A) 1.513, 1.503, 1.522 = 1.513
B) 1.513, 1.511, 1.450 = 1.491
C) 1.513, 1.459, 1.533 = 1.502
D) 1.513, 1.517, 1.512 = 1.514
E) 1.513, 1.510, 1.523 = 1.515
A) 1.513, 1.503, 1.522 = 1.513
B) 1.513, 1.511, 1.450 = 1.491
C) 1.513, 1.459, 1.533 = 1.502
D) 1.513, 1.517, 1.512 = 1.514
E) 1.513, 1.510, 1.523 = 1.515

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
Which statement A-D about accuracy and precision is not correct?
A) Precision refers to the reproducibility of repeated measurements.
B) Accuracy refers to how close a measured value is to the true value.
C) It is possible for measurements to be precise but not accurate.
D) Accuracy is determined by comparison with some standard.
E) Statements A-D all are correct.
A) Precision refers to the reproducibility of repeated measurements.
B) Accuracy refers to how close a measured value is to the true value.
C) It is possible for measurements to be precise but not accurate.
D) Accuracy is determined by comparison with some standard.
E) Statements A-D all are correct.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
If the following arithmetic operations were carried out on measured quantities, to how many significant figures should the answer be reported?
(5.70*16.90) / 7.2356
A) 1
B) 2
C) 3
D) 4
E) 5
(5.70*16.90) / 7.2356
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
The concentration (in % by volume) of methyl tert-butyl ether (MTBE) was determined in four samples of the same gasoline. What is the average value, and which measurement was the most accurate, compared to the average?
A) 5.05, sample 1
B) 5.05, sample 2
C) 5.05, sample 3
D) 5.05, sample 4
E) 5.0525, sample 3
A) 5.05, sample 1
B) 5.05, sample 2
C) 5.05, sample 3
D) 5.05, sample 4
E) 5.0525, sample 3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
How many 100 mg tablets can be produced from 100 kg of a pharmaceutical product?
A) 1,000
B) 10,000
C) 100,000
D) 1,000,000
E) 10,000,000
A) 1,000
B) 10,000
C) 100,000
D) 1,000,000
E) 10,000,000

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
A burette (shown below) was used to add dilute hydrochloric acid (HCl) to a solution containing sodium hydroxide (NaOH). If the burette initially was read as 0.00 mL, how much HCl has been delivered according to the reading in the figure? 
A) 5.4 mL
B) 5.40 mL
C) 4.60 mL
D) 4.3 mL
E) 4.30 mL

A) 5.4 mL
B) 5.40 mL
C) 4.60 mL
D) 4.3 mL
E) 4.30 mL

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
Electromagnetic radiation in the mid-infrared region of the spectrum has wavelengths around 0.6 m. Express this wavelength in meters using exponential notation (1 m = 10-6 m).
A) 1.06 *10-6 m
B) 1.06 *10-5 m
C) 1.06 m
D) 1.06 F*107 m
E) 1.06 *105 m
A) 1.06 *10-6 m
B) 1.06 *10-5 m
C) 1.06 m
D) 1.06 F*107 m
E) 1.06 *105 m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
As a summer intern at the National Institute of Standards and Technology, a student performed three measurements to determine the density of water at 25 C to four significant figures. She obtained the following results. The known density of water at 25 C to three significant figures is 0.958 g/mL. The measurements were __________
A) sufficiently precise but not accurate.
B) sufficiently accurate but not precise.
C) both sufficiently precise and accurate.
D) neither sufficiently precise nor accurate.
E) not repeated an adequate number of times.
A) sufficiently precise but not accurate.
B) sufficiently accurate but not precise.
C) both sufficiently precise and accurate.
D) neither sufficiently precise nor accurate.
E) not repeated an adequate number of times.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following quantities has two significant figures?
A) 0.4
B) 101
C) 1.10 * 103
D) 0.0092
E) 0.520
A) 0.4
B) 101
C) 1.10 * 103
D) 0.0092
E) 0.520

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
An irregularly shaped metal object with a mass of 25.43 g was placed in a graduated cylinder with water. The before and after volumes are shown below. What is the density of the metal? 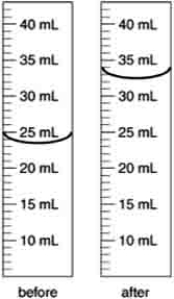
A) 2.8 g/cm3
B) 2.906 g/cm3
C) 0.782 g/cm3
D) 0.344 g/cm3
E) 2.734 g/cm3

A) 2.8 g/cm3
B) 2.906 g/cm3
C) 0.782 g/cm3
D) 0.344 g/cm3
E) 2.734 g/cm3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
Which one of the following values A-D contains four significant figures? If all do, choose E.
A) 0.0004 kg
B) 0.0040 L
C) 0.0400 m
D) 0.4000 mm
E) All contain four significant figures.
A) 0.0004 kg
B) 0.0040 L
C) 0.0400 m
D) 0.4000 mm
E) All contain four significant figures.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
What would you report for the total mass of three samples weighing 106.2 g, 33.15 g, and 0.028 g?
A) 139 g
B) 139.3 g
C) 139.4 g
D) 139.38 g
E) 139.378 g
A) 139 g
B) 139.3 g
C) 139.4 g
D) 139.38 g
E) 139.378 g

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
The diameter of the sun is 1,390,000 km. In scientific notation this is
A) 1.39 *10-6 km
B) 1.39*10-3 km
C) 1.39 * 106 km
D) 1.39* 103 km
E) 1.39 * 108 m
A) 1.39 *10-6 km
B) 1.39*10-3 km
C) 1.39 * 106 km
D) 1.39* 103 km
E) 1.39 * 108 m

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
As a summer intern at the National Institute of Standards and Technology, a student performed three measurements to determine the density of water at 25 C to four significant figures. She obtained the following results. The known density of water at 25 C to three significant figures is 0.958 g/mL. The measurements were __________
A) sufficiently precise but not accurate.
B) sufficiently accurate but not precise.
C) both sufficiently precise and accurate.
D) neither sufficiently precise nor accurate.
E) not repeated an adequate number of times.
A) sufficiently precise but not accurate.
B) sufficiently accurate but not precise.
C) both sufficiently precise and accurate.
D) neither sufficiently precise nor accurate.
E) not repeated an adequate number of times.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
Medicines usually are dispensed in units of mg. What is the mass of a 50 mg tablet?
A) 50 grams
B) 5.0 grams
C) 0.50 grams
D) 0.050 grams
E) 0.0050 grams
A) 50 grams
B) 5.0 grams
C) 0.50 grams
D) 0.050 grams
E) 0.0050 grams

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
If the following arithmetic operations are carried out, how many significant figures should be reported in the answer?
132)0 + 0.56 + 0.01 + 3.33
A) 1
B) 2
C) 3
D) 4
E) 5
132)0 + 0.56 + 0.01 + 3.33
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


