Exam 1: Matter and Energy: The Origin of the Universe
Exam 1: Matter and Energy: The Origin of the Universe99 Questions
Exam 2: Atoms, Ions, and Molecules: Matter Starts Here131 Questions
Exam 3: Stoichiometry: Mass, Formulas, and Reactions133 Questions
Exam 4: Solution Chemistry: The Hydrosphere126 Questions
Exam 5: Thermochemistry: Energy Changes in Reactions132 Questions
Exam 6: Properties of Gases: the Air We Breathe138 Questions
Exam 7: A Quantum Model of Atoms: Waves and Particles143 Questions
Exam 8: Chemical Bonds: What Makes a Gas a Greenhouse Gas139 Questions
Exam 9: Molecular Geometry: Shape Determines Function136 Questions
Exam 10: Intermolecular Forces: The Uniqueness of Water140 Questions
Exam 11: Solutions: Properties and Behavior130 Questions
Exam 12: Solids: Structures and Applications144 Questions
Exam 13: Organic Chemistry: Fuels, Pharmaceuticals, Materials, and Life129 Questions
Exam 14: Chemical Kinetics: Reactions in the Air We Breathe164 Questions
Exam 15: Chemical Equilibrium: How Much Product Does a Reaction Really Make91 Questions
Exam 16: Acid-Base and Solubility Equilibria: Reactions in Soil and Water179 Questions
Exam 17: Metal Ions: Colorful and Essential144 Questions
Exam 18: Thermodynamics: Spontaneous and Nonspontaneous Reactions and Processes157 Questions
Exam 19: Electrochemistry: the Quest for Clean Energy143 Questions
Exam 20: Biochemistry: the Compounds of Life108 Questions
Exam 21: Nuclear Chemistry: Applications to Energy and Medicine144 Questions
Exam 22: Life and the Periodic Table95 Questions
Select questions type
Which of the following represents a chemical property of copper metal?
Free
(Multiple Choice)
4.8/5  (42)
(42)
Correct Answer:
B
Which of the following represents a physical property of water?
Free
(Multiple Choice)
4.9/5  (22)
(22)
Correct Answer:
A
An irregularly shaped metal object with a mass of 25.43 g was placed in a graduated cylinder with water. The before and after volumes are shown below. What is the density of the metal? 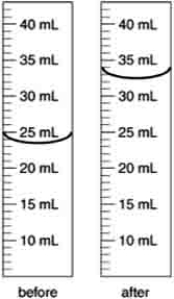
Free
(Multiple Choice)
4.9/5  (39)
(39)
Correct Answer:
A
Which statement correctly describes the properties of a gas?
(Multiple Choice)
4.9/5  (40)
(40)
This problem is from a New York Times article. Researchers tested a group of 28 doctors. The doctors were told that a 5-year-old child suffering a potentially fatal allergic reaction to peanuts needed an emergency injection of 0.12 mg of epinephrine. The bottle of epinephrine is labeled 1 mg in 1 mL of solution. What volume of this solution would you inject if you were the doctor?
(Multiple Choice)
4.8/5  (36)
(36)
In the movie The Italian Job, thieves steal gold bullion. One plan was to carry the ingots of gold off in suitcases. If each suitcase were 24 inches *18 inches*12 inches, approximately how much would each suitcase weigh when filled with gold? The molar mass of gold is 197 g/mol, the density of gold is 19.3 g/cm3, 1 in = 2.54 cm, and 1 kg = 2.20 lb.
(Multiple Choice)
4.8/5  (31)
(31)
Molecules are represented in various ways. Which statement A-D about molecular representations is not correct.
(Multiple Choice)
4.7/5  (36)
(36)
How thick is a square piece of aluminum foil that measures 5 cm on each side and has a mass of
675 mg? The density of aluminum is 2.70 g/cm3.
(Multiple Choice)
4.7/5  (38)
(38)
Usain Bolt holds the world record for the 100 m dash at 9.58 s. What was his average speed in miles/hour for this distance? The equivalence statements are 1 km = 0.621 mi and 3,600 s = 1 hr. It is interesting to compare this value with the average speed for walking (3 mph), bicycling (12 mph), and horse racing (38 mph).
(Multiple Choice)
4.9/5  (36)
(36)
At what temperature do the Celsius and Fahrenheit scales read the same?
(Multiple Choice)
4.9/5  (40)
(40)
In 1 second, light can travel 2.998 * 108 m. How many inches does light travel in 1 femtosecond
(1 fs = 10-15 s, 1 inch = 2.54 cm exactly)?
(Multiple Choice)
4.9/5  (43)
(43)
Which of the following mixtures can be separated by filtration?
(Multiple Choice)
4.9/5  (36)
(36)
Which one of the following is not equal to exactly one cubic meter (1 m3)?
(Multiple Choice)
4.7/5  (41)
(41)
The density of iron is 7.9 g/cm3. What is the volume of a 4.5 kg iron block?
(Multiple Choice)
4.8/5  (46)
(46)
Cheetahs can run at speeds of up to 60 mi per hour. How many seconds does it take a cheetah to run 10 m at this speed? (1 mi = 1.609 km)
(Multiple Choice)
4.9/5  (34)
(34)
Liquid nitrogen boils at 77 K. What is this temperature in °F?
(Multiple Choice)
4.9/5  (43)
(43)
On a summer day, the temperature in Phoenix, Arizona, was recorded as 110°F. What is this temperature in °C?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 1 - 20 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)