Deck 11: Properties of Solutions Their Concentrations and Colligative Properties
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/130
Play
Full screen (f)
Deck 11: Properties of Solutions Their Concentrations and Colligative Properties
1
Which solution will have the lowest osmotic pressure when measured against pure water?
A)0.10 M sodium chloride
B)0.10 M sodium sulfate
C)0.10 M sodium sulfide
D)0.10 M sodium phosphate
E)0.10 M sodium carbonate
A)0.10 M sodium chloride
B)0.10 M sodium sulfate
C)0.10 M sodium sulfide
D)0.10 M sodium phosphate
E)0.10 M sodium carbonate
0.10 M sodium chloride
2
Identify the following statement as true or false and choose the correct explanation: "For solutions requiring the same external pressure to cause reverse osmosis at the same temperature, the molarity of a sodium chloride solution will always be less than the molarity of a calcium chloride solution."
A)True, because the molar mass of sodium chloride is smaller.
B)True, because the van 't Hoff factor is smaller for sodium chloride.
C)False, because the van 't Hoff factor is smaller for sodium chloride.
D)False, because the van 't Hoff factor is larger for sodium chloride.
E)True, because the van 't Hoff factor is larger for sodium chloride.
A)True, because the molar mass of sodium chloride is smaller.
B)True, because the van 't Hoff factor is smaller for sodium chloride.
C)False, because the van 't Hoff factor is smaller for sodium chloride.
D)False, because the van 't Hoff factor is larger for sodium chloride.
E)True, because the van 't Hoff factor is larger for sodium chloride.
False, because the van 't Hoff factor is smaller for sodium chloride.
3
Drinking seawater is dehydrating to humans because
A)there is a net flow of water molecules out of the cells because the solution is hypertonic.
B)there is a net flow of water molecules out of the cells because the solution is hypotonic.
C)there is a net flow of hydrated Na+, Cl-, and other ions into the cells because the solution is hypertonic.
D)there is a net flow of hydrated Na+, Cl-, and other ions into the cells because the solution is hypotonic.
E)the hydrated Na+, Cl-, and other ions block channels in the cell membrane, preventing water molecules from migrating into or out of the cells.
A)there is a net flow of water molecules out of the cells because the solution is hypertonic.
B)there is a net flow of water molecules out of the cells because the solution is hypotonic.
C)there is a net flow of hydrated Na+, Cl-, and other ions into the cells because the solution is hypertonic.
D)there is a net flow of hydrated Na+, Cl-, and other ions into the cells because the solution is hypotonic.
E)the hydrated Na+, Cl-, and other ions block channels in the cell membrane, preventing water molecules from migrating into or out of the cells.
there is a net flow of water molecules out of the cells because the solution is hypertonic.
4
Two solutions, A and B, are separated by a semipermeable membrane.Which of the following statements regarding their tonicities is NOT correct? Assume the same van 't Hoff factor for each solution.
A)If A and B are of equal molarities, they are isotonic.
B)If the molarity of A is lower than that of B, solvent will flow from B to A.
C)If the molarity of B is lower than that of A, B is hypotonic relative to A.
D)If the molarity of A is lower than that of B, the osmotic pressure of B is higher than that of A.
E)If the molarity of B is lower than that of A, A is hypertonic relative to B.
A)If A and B are of equal molarities, they are isotonic.
B)If the molarity of A is lower than that of B, solvent will flow from B to A.
C)If the molarity of B is lower than that of A, B is hypotonic relative to A.
D)If the molarity of A is lower than that of B, the osmotic pressure of B is higher than that of A.
E)If the molarity of B is lower than that of A, A is hypertonic relative to B.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
5
The freezing point of a 0.0925 m solution of ammonium chloride was found to be -0.325 C.What is the actual van 't Hoff factor for this salt at this concentration? (Kf (water) =1.86 C/m)
A)1.89
B)1.95
C)1.86
D)1.80
E)1.97
A)1.89
B)1.95
C)1.86
D)1.80
E)1.97

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following regarding colligative properties of a solution is NOT correct?
A)Colligative properties depend on the concentration of the particles dissolved in the solvent.
B)Vapor pressure reduction is a colligative property.
C)The temperature range over which a solution remains liquid is larger than that of the pure solvent due to the presence of solute particles.
D)The change in the boiling point of a solvent due to the presence of a solute is a colligative property.
E)The freezing point of a solution increases as the concentration of solute increases.
A)Colligative properties depend on the concentration of the particles dissolved in the solvent.
B)Vapor pressure reduction is a colligative property.
C)The temperature range over which a solution remains liquid is larger than that of the pure solvent due to the presence of solute particles.
D)The change in the boiling point of a solvent due to the presence of a solute is a colligative property.
E)The freezing point of a solution increases as the concentration of solute increases.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
7
What is the osmotic pressure of a 0.300 M blood plasma sample at 37.0 C? Assume a van't Hoff factor of 1.
A)1.34 atm
B)9.23 atm
C)7.34 atm
D)14.8 atm
E)7.64 atm
A)1.34 atm
B)9.23 atm
C)7.34 atm
D)14.8 atm
E)7.64 atm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following solutions is isotonic with the solution inside a red blood cell, which has an osmotic pressure of 7.89 atm at 37.0 C?
A)0.155 M glucose (C6H12O6)
B)0.310 M sodium acetate (NaCH3COO)
C)0.155 M sodium phosphate (Na3PO4)
D)0.155 M potassium chloride (KCl)
E)0.310 M calcium chloride (CaCl2)
A)0.155 M glucose (C6H12O6)
B)0.310 M sodium acetate (NaCH3COO)
C)0.155 M sodium phosphate (Na3PO4)
D)0.155 M potassium chloride (KCl)
E)0.310 M calcium chloride (CaCl2)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
9
In the process of dialysis, a special semipermeable membrane allows both small molecules and water to pass through, but not large protein molecules.These membranes are used to separate these small molecules and ions from much larger proteins.If a mixture of proteins and small molecules were separated from pure water by a dialysis membrane as shown in the figure, which way would the molecules flow? 
A)Both water and small molecules would pass through into the pure water.
B)Only the proteins would pass through into the pure water.
C)Water would enter the solution while small molecules would pass through into the pure water.
D)Only small molecules would pass through the membrane; water and proteins would not.
E)Only water would pass through the membrane into the solution.

A)Both water and small molecules would pass through into the pure water.
B)Only the proteins would pass through into the pure water.
C)Water would enter the solution while small molecules would pass through into the pure water.
D)Only small molecules would pass through the membrane; water and proteins would not.
E)Only water would pass through the membrane into the solution.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
10
Calculate the minimum pressure that must be applied to achieve reverse osmosis of 0.320 M KBr at 25.0 C.
A)1.31 atm
B)133 atm
C)1590 atm
D)7.82 atm
E)15.7 atm
A)1.31 atm
B)133 atm
C)1590 atm
D)7.82 atm
E)15.7 atm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements regarding solutions and the van 't Hoff factor is NOT correct?
A)Nonideal behavior can occur in solutions containing ions when the ions do not behave as independent particles in solution.
B)Theoretical van 't Hoff factors often predict colligative properties of solutions with concentrations of about 0.01 m or less.
C)In dilute solutions, the experimental van 't Hoff factor for a molecule is always greater than 1.
D)As concentrations increase, the probability of ion pairing increases.
E)The deviation from ideal behavior generally increases as the charge on dissolved ions increases.
A)Nonideal behavior can occur in solutions containing ions when the ions do not behave as independent particles in solution.
B)Theoretical van 't Hoff factors often predict colligative properties of solutions with concentrations of about 0.01 m or less.
C)In dilute solutions, the experimental van 't Hoff factor for a molecule is always greater than 1.
D)As concentrations increase, the probability of ion pairing increases.
E)The deviation from ideal behavior generally increases as the charge on dissolved ions increases.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
12
Which solution, if either, would create the higher osmotic pressure compared to pure water): one prepared from 1.0 g of NaCl in 10 mL of water or one prepared from 1.0 g of CsBr in 10 mL of water?
A)They would have the same osmotic pressures.
B)NaCl would give the higher pressure.
C)CsBr would give the higher pressure.
D)It is impossible to tell.
E)These compounds are salts and do not produce an osmotic pressure.
A)They would have the same osmotic pressures.
B)NaCl would give the higher pressure.
C)CsBr would give the higher pressure.
D)It is impossible to tell.
E)These compounds are salts and do not produce an osmotic pressure.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
13
Identify the following statement as true or false and choose the correct explanation: "For solutions with the same molarity at the same temperature, the pressure needed for reverse osmosis of a sodium chloride solution will always be less than the pressure needed for a calcium chloride solution."
A)True, because the molar mass of sodium chloride is smaller.
B)True, because the van 't Hoff factor is smaller for sodium chloride.
C)False, because the van 't Hoff factor is smaller for sodium chloride.
D)False, because the van 't Hoff factor is larger for sodium chloride.
E)True, because the van 't Hoff factor is larger for sodium chloride.
A)True, because the molar mass of sodium chloride is smaller.
B)True, because the van 't Hoff factor is smaller for sodium chloride.
C)False, because the van 't Hoff factor is smaller for sodium chloride.
D)False, because the van 't Hoff factor is larger for sodium chloride.
E)True, because the van 't Hoff factor is larger for sodium chloride.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
14
A solution of 5.00 g of sodium chloride in 1.00 kg of water has a freezing point of -0.299 C.What is the actual van 't Hoff factor for this salt at this concentration? (Kf (water) = 1.86 C/m)
A)1.88
B)1.98
C)1.93
D)1.83
E)1.94
A)1.88
B)1.98
C)1.93
D)1.83
E)1.94

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
15
What is the osmotic pressure of a 5.20% mass/volume aqueous ethanol (CH3CH2OH, 46.07 g/mol) solution at 25.0 C? Assume the density of the solution is 1.00 g/mL.
A)2.32 atm
B)27.6 atm
C)2.80 * 103 atm
D)58.6 atm
E)4.91 atm
A)2.32 atm
B)27.6 atm
C)2.80 * 103 atm
D)58.6 atm
E)4.91 atm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
16
Which statement is NOT correct? Determination of the molar mass of an unknown sample by an osmotic pressure measurement requires that
A)the solute dissolved in the solution is pure.
B)the solute is a nonelectrolyte.
C)the molecules of the solute do not pass through the semipermeable membrane.
D)the mass of the solute dissolved in the solution is known in advance.
E)the molar concentration of the solute in the solution is known in advance.
A)the solute dissolved in the solution is pure.
B)the solute is a nonelectrolyte.
C)the molecules of the solute do not pass through the semipermeable membrane.
D)the mass of the solute dissolved in the solution is known in advance.
E)the molar concentration of the solute in the solution is known in advance.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
17
Which statement regarding osmotic pressure is NOT correct? Osmotic pressure
A)increases with increasing temperature.
B)increases with increasing molar concentration of the solute.
C)is greater for 0.1 M Na2SO4 than for 0.1 M NaCl.
D)depends on the value of the gas constant R.
E)is the same for solutions with the same mass percent of solute.
A)increases with increasing temperature.
B)increases with increasing molar concentration of the solute.
C)is greater for 0.1 M Na2SO4 than for 0.1 M NaCl.
D)depends on the value of the gas constant R.
E)is the same for solutions with the same mass percent of solute.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
18
What is the osmotic pressure of a 0.0540 M aqueous sodium sulfate (Na2SO4, 142.04 g/mol) solution at 25.0 C?
A)1.32 atm
B)2.64 atm
C)3.96 atm
D)134 atm
E)33.7 atm
A)1.32 atm
B)2.64 atm
C)3.96 atm
D)134 atm
E)33.7 atm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
19
What is the molarity of a sucrose (C12H22O11) solution that produces an osmotic pressure of 2.65 atm at 25.0 C?
A)0.0349 M
B)0.127 M
C)0.0127 M
D)0.108 M
E)0.398 M
A)0.0349 M
B)0.127 M
C)0.0127 M
D)0.108 M
E)0.398 M

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
20
What would be the freezing point of a 1.50 m solution of NaNO3 in water? (Kf = 1.86 C)
A)5.58 C
B)(-2.79 C)
C)(-5.58 C)
D)2.79 C
E)(-1.61 C)
A)5.58 C
B)(-2.79 C)
C)(-5.58 C)
D)2.79 C
E)(-1.61 C)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
21
A 0.512 g sample of an unknown nondissociating solute was dissolved in 25.0 g of camphor (Kf = 39.7 C/m), decreasing the freezing point of camphor by 3.07 C.What is the molar mass of the solute?
A)150 g/mol
B)166 g/mol
C)265 g/mol
D)631 g/mol
E)323 g/mol
A)150 g/mol
B)166 g/mol
C)265 g/mol
D)631 g/mol
E)323 g/mol

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
22
Which statement below regarding the liquid-gas phase transition and boiling is NOT correct?
A)More molecules have sufficient energy to escape the liquid phase at higher temperatures.
B)The vapor pressure of a liquid increases with temperature.
C)Boiling occurs only when the vapor pressure of a liquid equals 1 standard atm.
D)More volatile liquids have lower boiling points.
E)Liquids with stronger intermolecular forces typically have higher boiling points.
A)More molecules have sufficient energy to escape the liquid phase at higher temperatures.
B)The vapor pressure of a liquid increases with temperature.
C)Boiling occurs only when the vapor pressure of a liquid equals 1 standard atm.
D)More volatile liquids have lower boiling points.
E)Liquids with stronger intermolecular forces typically have higher boiling points.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
23
A physiological saline solution is 0.92% NaCl by mass.What is the osmotic pressure of such a solution at a body temperature of 37 C? Assume the density of the solution is 1.0 g/mL.
A)8.0 atm
B)3.9 atm
C)2.3 atm
D)4.3 atm
E)4.1 atm
A)8.0 atm
B)3.9 atm
C)2.3 atm
D)4.3 atm
E)4.1 atm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
24
A 15.0 mg sample of a protein was dissolved in water to produce 5.00 mL of solution at 25.1 C.The osmotic pressure of this solution was measured and found to be 6.50 torr.What is the molar mass of this protein?
A)427 g/mol
B)941 g/mol
C)8580 g/mol
D)4270 g/mol
E)561 g/mol
A)427 g/mol
B)941 g/mol
C)8580 g/mol
D)4270 g/mol
E)561 g/mol

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
25
You have learned that adding table salt, NaCl (58.44 g/mol, 2.16 g/cm3), to water (Kb = 0.52oC/m) increases the temperature at which it boils.You decide to try cooking pasta faster at a higher temperature in boiling salty water.What increase in the boiling point do you expect if you add 4 tablespoons (1 tbsp = 14.8 cm3) of salt to 16 oz of water (474 mL)?
A)14 C
B)4.8 C
C)28 C
D)3.0 C
E)8.4 C
A)14 C
B)4.8 C
C)28 C
D)3.0 C
E)8.4 C

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
26
You wish to de-ice your driveway, so you consider buying 10 lb of NaCl, 10 lb of CaCl2, or 10 lb of MgCl2.Which of these would de-ice your driveway most effectively? Assume that ideal dissociation of the salts occurs and that colligative properties are the only consideration.
A)10 lb of NaCl will de-ice best.
B)10 lb of CaCl2 will de-ice best.
C)10 lb of MgCl2 will de-ice best.
D)The three driveways will de-ice to the same extent.
E)10 lb of CaCl2 or 10 lb of MgCl2 will de-ice equally well.
A)10 lb of NaCl will de-ice best.
B)10 lb of CaCl2 will de-ice best.
C)10 lb of MgCl2 will de-ice best.
D)The three driveways will de-ice to the same extent.
E)10 lb of CaCl2 or 10 lb of MgCl2 will de-ice equally well.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
27
Cholesterol is poorly soluble in water, but it will dissolve in nonpolar solvents such as cyclohexane (C6H12, 84.18 g/mol, Kf = 20.2 C/m, Tf = 6.55 C).When 10.0 g cholesterol was dissolved in 0.500 kg cyclohexane, the freezing point of the solution was 5.50 C.What is the approximate molar mass of cholesterol?
A)404 g/mol
B)385 g/mol
C)73.5 g/mol
D)248 g/mol
E)424 g/mol
A)404 g/mol
B)385 g/mol
C)73.5 g/mol
D)248 g/mol
E)424 g/mol

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
28
The normal boiling point of bromine is 58.8 C, and its enthalpy of vaporization is 30.91 kJ/mol.What is the approximate vapor pressure of bromine at 10.0 C?
A)0.998 atm
B)0.735 atm
C)0.853 atm
D)0.145 atm
E)6.89 atm
A)0.998 atm
B)0.735 atm
C)0.853 atm
D)0.145 atm
E)6.89 atm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
29
Which statement below regarding evaporation and condensation of a liquid in a closed container is NOT correct?
A)When a liquid is first placed in a closed container, the evaporation rate is higher than it is in an open container under the same conditions.
B)When a liquid is first placed in a closed container, the evaporation rate is higher than the condensation rate.
C)When the rates of evaporation and condensation are equal, the system has reached dynamic equilibrium.
D)Vapor pressure refers to the pressure of a gas at a given temperature in equilibrium with its liquid phase.
E)Stronger intermolecular forces within the liquid typically result in a lower vapor pressure under a given set of conditions.
A)When a liquid is first placed in a closed container, the evaporation rate is higher than it is in an open container under the same conditions.
B)When a liquid is first placed in a closed container, the evaporation rate is higher than the condensation rate.
C)When the rates of evaporation and condensation are equal, the system has reached dynamic equilibrium.
D)Vapor pressure refers to the pressure of a gas at a given temperature in equilibrium with its liquid phase.
E)Stronger intermolecular forces within the liquid typically result in a lower vapor pressure under a given set of conditions.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
30
Which statement below regarding evaporation is NOT correct?
A)A liquid in an open container will evaporate because some molecules will have enough kinetic energy to escape into the vapor phase.
B)Stronger intermolecular forces enable more molecules to escape from the liquid phase into the vapor phase.
C)Higher temperatures promote higher evaporation rates because more molecules have enough kinetic energy to escape into the vapor phase.
D)A solid can have a vapor pressure just as a liquid does.
E)Potential energy changes accompany kinetic energy changes when a liquid evaporates.
A)A liquid in an open container will evaporate because some molecules will have enough kinetic energy to escape into the vapor phase.
B)Stronger intermolecular forces enable more molecules to escape from the liquid phase into the vapor phase.
C)Higher temperatures promote higher evaporation rates because more molecules have enough kinetic energy to escape into the vapor phase.
D)A solid can have a vapor pressure just as a liquid does.
E)Potential energy changes accompany kinetic energy changes when a liquid evaporates.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
31
You have learned that adding table salt, NaCl (58.44 g/mol, 2.16 g/cm3), to water (Kb = 0.52 C/m) increases the temperature at which it boils.You decide to try cooking pasta faster at a higher temperature in boiling salty water.What increase in the boiling point do you expect if you add 1 tablespoon (1 tbsp = 14.8 cm3) of salt to one 8 oz cup of water (237 mL)?
A)1.0 C
B)2.4 C
C)28 C
D)1.5 C
E)3.7 C
A)1.0 C
B)2.4 C
C)28 C
D)1.5 C
E)3.7 C

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
32
A 1.00 L aqueous magnesium chloride solution has an osmotic pressure of 15.4 atm at 25 C.How many grams of MgCl2 (95.21 g/mol) were required to make this solution?
A)59.9 g
B)151 g
C)714 g
D)1.77 g
E)20.0 g
A)59.9 g
B)151 g
C)714 g
D)1.77 g
E)20.0 g

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
33
Which statement below regarding vapor pressure is NOT correct?
A)Vapor pressure is an intensive property.
B)The substance with the stronger intermolecular forces has the lower vapor pressure.
C)Vapor pressure increases with increasing temperature.
D)Pure water has a higher vapor pressure at a given temperature than seawater.
E)A nonvolatile solute increases the vapor pressure of the solvent.
A)Vapor pressure is an intensive property.
B)The substance with the stronger intermolecular forces has the lower vapor pressure.
C)Vapor pressure increases with increasing temperature.
D)Pure water has a higher vapor pressure at a given temperature than seawater.
E)A nonvolatile solute increases the vapor pressure of the solvent.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
34
A 275 mg sample of a nonelectrolyte compound was dissolved in water to produce 10.0 mL of a solution at 25 C.The osmotic pressure of this solution was measured and found to be 3.67 atm.What is the molar mass of this compound?
A)183 g/mol
B)18.3 g/mol
C)101 g/mol
D)1560 g/mol
E)1830 g/mol
A)183 g/mol
B)18.3 g/mol
C)101 g/mol
D)1560 g/mol
E)1830 g/mol

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
35
Magnesium chloride is often used to melt ice on sidewalks.Considering that the solubility of magnesium chloride 95.21 (g/mol) in water is 54.3 g per 100.0 g of water, what is the lowest temperature that you would expect to be able to melt ice with magnesium chloride? Assume ideal behavior.(Kf (water) =1.86 C/m)
A)(-41.0 C)
B)(-31.8 C)
C)(-10.6 C)
D)(-25.0 C)
E)(-29.2 C)
A)(-41.0 C)
B)(-31.8 C)
C)(-10.6 C)
D)(-25.0 C)
E)(-29.2 C)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
36
Intravenously administered saline solution must have an osmotic pressure that matches that of blood to prevent hemolysis or crenation of blood cells.What mass of sodium chloride (58.44 g/mol) is needed to produce 100.0 mL of saline solution with an osmotic pressure of 7.83 atm at a body temperature of 37 C?
A)0.899 g
B)8.99 g
C)18.0 g
D)1.80 g
E)0.450 g
A)0.899 g
B)8.99 g
C)18.0 g
D)1.80 g
E)0.450 g

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following would be most effective in melting ice on a sidewalk?
A)1 kg NaCl
B)1 kg KCl
C)1 kg MgCl2
D)1 kg CuCl
E)1 kg CaCl2
A)1 kg NaCl
B)1 kg KCl
C)1 kg MgCl2
D)1 kg CuCl
E)1 kg CaCl2

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
38
Myristicin is a hallucinogenic compound found naturally in nutmeg.A 425 mg sample of myristicin was dissolved in 7.800 g of camphor (Kf = 39.7 C/m), decreasing the freezing point of camphor by 11.3 C.What is the molar mass of myristicin?
A)65.0 g/mol
B)130.g/mol
C)191 g/mol
D)451 g/mol
E)1070 g/mol
A)65.0 g/mol
B)130.g/mol
C)191 g/mol
D)451 g/mol
E)1070 g/mol

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
39
You are working as a research intern in a biochemistry lab, and you synthesize a new protein.You decide to use osmosis to determine your protein's molar mass.You dissolve 0.120 g of the protein in water to make 20.00 mL of solution, and the osmotic pressure is 3.53 torr at 298 K.You report that the molar mass is g/mol.
A)between 0 and 1000
B)between 1000 and 5000
C)between 5000 and 10,000
D)between 10,000 and 20,000
E)between 20,000 and 50,000
A)between 0 and 1000
B)between 1000 and 5000
C)between 5000 and 10,000
D)between 10,000 and 20,000
E)between 20,000 and 50,000

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following aqueous solutions will have the lowest freezing point?
A)0.20 m ethanol, CH3CH2OH
B)0.30 m potassium chloride, KCl
C)0.15 m calcium chloride, CaCl2
D)0.10 m sodium carbonate, Na2CO3
E)0.25 m sodium nitrate, NaNO3
A)0.20 m ethanol, CH3CH2OH
B)0.30 m potassium chloride, KCl
C)0.15 m calcium chloride, CaCl2
D)0.10 m sodium carbonate, Na2CO3
E)0.25 m sodium nitrate, NaNO3

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
41
A solution is prepared by mixing 75 g of methanol (CH3OH, 32.04 g/mol) with 25 g of ethanol (CH3CH2OH, 46.07 g/mol).What is the partial pressure of methanol in the vapor phase at 20 C? 
A)92 torr
B)83 torr
C)75 torr
D)55 torr
E)45 torr

A)92 torr
B)83 torr
C)75 torr
D)55 torr
E)45 torr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
42
What physical property is used to separate the hydrocarbon components in petroleum crude oil)?
A)melting point
B)density
C)boiling point
D)molar mass
E)viscosity
A)melting point
B)density
C)boiling point
D)molar mass
E)viscosity

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
43
Which statement regarding nonideal solutions is NOT correct?
A)The boiling point of a nonideal solution is always higher than that of an ideal solution.
B)Ideal solutions follow Raoult's law, where the total vapor pressure is the sum of the mole fractions of the volatile components multiplied by their vapor pressure when they are pure (Ptotal =x1P1 + x2P2 +…).
C)When adhesive forces are stronger than cohesive forces in the solution, negative deviations from Raoult's law are observed.
D)Positive deviations from Raoult's law are observed when solute-solute and solvent-solvent interactions are stronger than solute-solvent attractions.
E)Raoult's law applies to mixtures of volatile components.
A)The boiling point of a nonideal solution is always higher than that of an ideal solution.
B)Ideal solutions follow Raoult's law, where the total vapor pressure is the sum of the mole fractions of the volatile components multiplied by their vapor pressure when they are pure (Ptotal =x1P1 + x2P2 +…).
C)When adhesive forces are stronger than cohesive forces in the solution, negative deviations from Raoult's law are observed.
D)Positive deviations from Raoult's law are observed when solute-solute and solvent-solvent interactions are stronger than solute-solvent attractions.
E)Raoult's law applies to mixtures of volatile components.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
44
A solution is prepared by mixing 45.68 g of carbon disulfide (CS2, 76.13 g/mol) with 16.42 g of acetonitrile (CH3CN, 41.06 g/mol).What is the mole fraction of CS2 in the vapor phase at 25 C? 
A)1.00
B)0.600
C)0.167
D)0.273
E)0.857

A)1.00
B)0.600
C)0.167
D)0.273
E)0.857

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
45
The smell of fresh-cut pine is due in part to a cyclic alkene called pinene.A graph of the natural logarithm of the vapor pressure of pinene vs.1/temperature produces a straight line with a slope of -4936.37 K.What is the enthalpy of vaporization of pinene?
A)(+397 kJ/mol)
B)(-39.7 kJ/mol)
C)(+39.7 kJ/mol)
D)(-41.0 kJ/mol)
E)(+41.0 kJ/mol)
A)(+397 kJ/mol)
B)(-39.7 kJ/mol)
C)(+39.7 kJ/mol)
D)(-41.0 kJ/mol)
E)(+41.0 kJ/mol)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
46
Thiophene, C4H4S, is a fairly volatile liquid with a vapor pressure of 40.0 torr at 12.5 C, and its normal boiling point is 84.0 C.Calculate an estimate of thiophene's enthalpy of vaporization in kJ/mol.
A)(-34.9 kJ/mol)
B)(+34.9 kJ/mol)
C)(+23.4 kJ/mol)
D)(-15.2 kJ/mol)
E)(+15.2 kJ/mol)
A)(-34.9 kJ/mol)
B)(+34.9 kJ/mol)
C)(+23.4 kJ/mol)
D)(-15.2 kJ/mol)
E)(+15.2 kJ/mol)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
47
Which statement regarding the boiling of a mixture of liquids A and B is NOT correct? 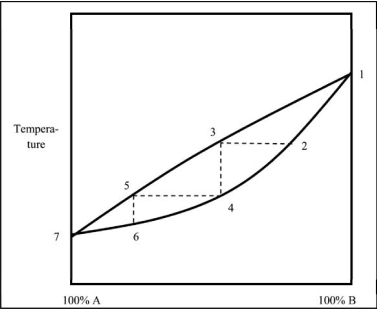
A)The distillate collected boils at lower temperatures as the percentage of A increases.
B)The distillate always contains a higher percentage of B because pure B is less volatile than pure A.
C)The upper curve reflects the composition of the vapor at a given temperature.
D)As the distillation progresses from point 2 to point 6, the liquid becomes richer in A.
E)The boiling point of pure A is lower than that of pure B.

A)The distillate collected boils at lower temperatures as the percentage of A increases.
B)The distillate always contains a higher percentage of B because pure B is less volatile than pure A.
C)The upper curve reflects the composition of the vapor at a given temperature.
D)As the distillation progresses from point 2 to point 6, the liquid becomes richer in A.
E)The boiling point of pure A is lower than that of pure B.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
48
A solution is prepared by mixing 45.68 g of carbon disulfide (CS2, 76.13 g/mol) with 16.42 g of acetonitrile (CH3CN, 41.06 g/mol).What is the partial pressure of CS2 in the vapor phase at 25 C? 
A)3.57 *102 torr
B)2.50 * 102 torr
C)2.14 * 102 torr
D)1.97 * 102 torr
E)8.95 * 101 torr

A)3.57 *102 torr
B)2.50 * 102 torr
C)2.14 * 102 torr
D)1.97 * 102 torr
E)8.95 * 101 torr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
49
Gasoline is primarily a mixture of hydrocarbons and is sold with an octane rating that is based on a comparison with the properties of isooctane (C8H18), which has an enthalpy of vaporization of 35.8 kJ/mol and a normal boiling point of 98.2 C.Determine the vapor pressure of isooctane on a very hot day when the temperature is 38.0 C.
A)80.6 torr
B)36.7 torr
C)67.8 torr
D)47.9 torr
E)89.3 torr
A)80.6 torr
B)36.7 torr
C)67.8 torr
D)47.9 torr
E)89.3 torr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
50
The aroma from almonds and cherries is due in part to a compound called benzaldehyde.A graph of the natural logarithm of the vapor pressure of benzaldehyde vs.1/temperature produces a straight line with a slope of -5870.99 K.What is the enthalpy of vaporization of benzaldehyde?
A)(+473 kJ/mol)
B)(-47.3 kJ/mol)
C)(+47.3 kJ/mol)
D)(-48.8 kJ/mol)
E)(+48.8 kJ/mol)
A)(+473 kJ/mol)
B)(-47.3 kJ/mol)
C)(+47.3 kJ/mol)
D)(-48.8 kJ/mol)
E)(+48.8 kJ/mol)

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
51
A solution is prepared by mixing 50.00 g of methanol (CH3OH, 32.04 g/mol) with 50.00 g of ethanol (CH3CH2OH, 46.07 g/mol).Use the following data to determine the vapor pressure of this solution at 20 C. 
A)69 torr
B)57 torr
C)79 torr
D)72 torr
E)83 torr

A)69 torr
B)57 torr
C)79 torr
D)72 torr
E)83 torr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
52
A solution is prepared by mixing 75 g of methanol (CH3OH, 32.04 g/mol) with 25 g of ethanol (CH3CH2OH, 46.07 g/mol).What is the mole fraction of ethanol in the vapor phase at 20 C? 
A)0.10
B)0.12
C)0.19
D)0.32
E)0.54

A)0.10
B)0.12
C)0.19
D)0.32
E)0.54

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
53
A solution is prepared by mixing 45.68 g of carbon disulfide (CS2, 76.13 g/mol) with 16.43 g of acetonitrile (CH3CN, 41.06 g/mol).By what factor is the vapor phase enriched in CS2 at 25 C? 
A)8.97
B)1.67
C)5.99
D)3.99
E)1.47

A)8.97
B)1.67
C)5.99
D)3.99
E)1.47

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
54
Lanterns and stoves that use n-pentane as a fuel are often difficult to light on a cold day because the fuel has a low vapor pressure at low temperatures.Determine the vapor pressure of n-pentane on a night when the temperature is 0.0 C.The enthalpy of vaporization of n-pentane is 27.6 kJ/mol, and its boiling point is 36.0 C.
A)228 torr
B)367 torr
C)185 torr
D)479 torr
E)209 torr
A)228 torr
B)367 torr
C)185 torr
D)479 torr
E)209 torr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
55
The normal boiling point of ammonia is -33.34 C, and its enthalpy of vaporization is 23.35 kJ/mol.What pressure would have to be applied for ammonia to boil at 25.00 C?
A)9.891 atm
B)0.09626 atm
C)0.9037 atm
D)1.268 atm
E)10.39 atm
A)9.891 atm
B)0.09626 atm
C)0.9037 atm
D)1.268 atm
E)10.39 atm

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
56
Gasoline is primarily a mixture of hydrocarbons and is sold with an octane rating that is based on a comparison with the combustion properties of isooctane.Gasoline usually contains an isomer of isooctane called tetramethylbutane (C8H18), which has an enthalpy of vaporization of 43.3 kJ/mol and a boiling point of 106.5 C.Determine the vapor pressure of tetramethylbutane on a very hot day when the temperature is 38.0 C.
A)80.0 torr
B)37.1 torr
C)67.8 torr
D)47.9 torr
E)89.3 torr
A)80.0 torr
B)37.1 torr
C)67.8 torr
D)47.9 torr
E)89.3 torr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
57
A solution is prepared by mixing 75 g of methanol (CH3OH, 32.04 g/mol) with 25 g of ethanol (CH3CH2OH, 46.07 g/mol).Use the following data to determine the vapor pressure of this solution at 20 C. 
A)69 torr
B)57 torr
C)80 torr
D)73 torr
E)83 torr

A)69 torr
B)57 torr
C)80 torr
D)73 torr
E)83 torr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
58
A solution is prepared by mixing 45.68 g of carbon disulfide (CS2, 76.13 g/mol) with 16.42 g of acetonitrile (CH3CN, 41.06 g/mol).What is the vapor pressure of the solution at 25 C? 
A)3.57* 102 torr
B)2.50 * 102 torr
C)2.14 *102 torr
D)1.97 * 102 torr
E)8.95 *101 torr

A)3.57* 102 torr
B)2.50 * 102 torr
C)2.14 *102 torr
D)1.97 * 102 torr
E)8.95 *101 torr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
59
Which statement regarding the fractional distillation of two liquids is NOT correct?
A)The substance with the lower boiling point has a higher concentration in the vapor than in the liquid.
B)The boiling point of the mixture is not constant, changing as the distillation progresses.
C)The substance with the higher vapor pressure has a higher concentration in the vapor than in the liquid.
D)The mole ratio of the two substances in the vapor is not the same as it is in the liquid.
E)Raoult's law does not apply to mixtures of volatile components.
A)The substance with the lower boiling point has a higher concentration in the vapor than in the liquid.
B)The boiling point of the mixture is not constant, changing as the distillation progresses.
C)The substance with the higher vapor pressure has a higher concentration in the vapor than in the liquid.
D)The mole ratio of the two substances in the vapor is not the same as it is in the liquid.
E)Raoult's law does not apply to mixtures of volatile components.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following pairs of liquids probably exhibits positive deviations from Raoult's law when mixed?
A)CH3OH and H2O
B)C6H14 and C6H6
C)CH3Br and H2O
D)CH3CH2OH and CH3COOH
E)C7H16 and C8H18
A)CH3OH and H2O
B)C6H14 and C6H6
C)CH3Br and H2O
D)CH3CH2OH and CH3COOH
E)C7H16 and C8H18

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
61
What is the vapor pressure of an aqueous solution that has a solute mole fraction of =0.200? The vapor pressure of water is 25.756 mmHg at 25 C.
A)20.6 mmHg
B)5.15 mmHg
C)25.556 mmHg
D)0.800 mmHg
E)30.9 mmHg
A)20.6 mmHg
B)5.15 mmHg
C)25.556 mmHg
D)0.800 mmHg
E)30.9 mmHg

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
62
You wish to prepare a solution of methanol (CH3OH, 32.04 g/mol) and ethanol (CH3CH2OH, 46.07 g/mol) that has a total vapor pressure of 66 torr at 20 C.Calculate the mole fraction of ethanol in the solution that will produce the desired pressure.

A)0.55
B)0.68
C)0.48
D)0.72
E)0.38

A)0.55
B)0.68
C)0.48
D)0.72
E)0.38

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
63
The concentration unit of molality is symbolized as
A)M
B)m
C)M.
D)mol
E)Mo
A)M
B)m
C)M.
D)mol
E)Mo

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following statements regarding the phase diagram of water and an aqueous solution is NOT correct? Temperature is on the x-axis; pressure is on the y-axis. 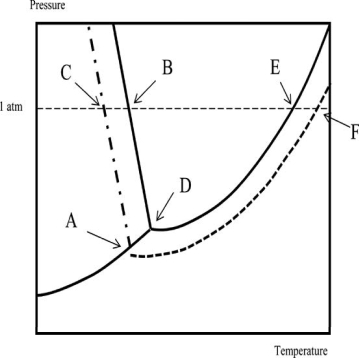
A)The boiling point of the solution is higher than that of the solvent by an amount indicated by the difference in temperature between points E and F.
B)Point D corresponds to the triple point of the solvent.
C)The solution boils at the temperature corresponding to point F.
D)The normal freezing point of the solution corresponds to point A.
E)The freezing point of the solvent is higher than that of the solution.

A)The boiling point of the solution is higher than that of the solvent by an amount indicated by the difference in temperature between points E and F.
B)Point D corresponds to the triple point of the solvent.
C)The solution boils at the temperature corresponding to point F.
D)The normal freezing point of the solution corresponds to point A.
E)The freezing point of the solvent is higher than that of the solution.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following pairs of liquids probably exhibits very nearly ideal behavior when mixed?
A)CH3NH2 and H2O
B)CH3CN and H2O
C)CH3Br and CH3OCH3
D)CH3CH2OH and CH3COOH
E)C7H16 and C8H18
A)CH3NH2 and H2O
B)CH3CN and H2O
C)CH3Br and CH3OCH3
D)CH3CH2OH and CH3COOH
E)C7H16 and C8H18

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
66
Calculate the molality of a solution containing 0.575 mol of ethanol (CH3CH2OH) and 395 g of water.
A)1.46* 10-3 m
B)1.46 m
C)0.687 m
D)0.227 m
E)227 m
A)1.46* 10-3 m
B)1.46 m
C)0.687 m
D)0.227 m
E)227 m

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
67
A solution is prepared by adding 1.50 mol of glucose, which is not volatile, to 3.50 mol of water.What is the vapor pressure of this solution at 25 C given that the vapor pressure of pure water is 23.8 torr?
A)7.00 torr
B)16.7 torr
C)10.2 torr
D)7.14 torr
E)34.0 torr
A)7.00 torr
B)16.7 torr
C)10.2 torr
D)7.14 torr
E)34.0 torr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
68
What is the boiling point of a solution prepared by dissolving 157.0 g of urea (CH4N2O, 60.06 g/mol) in 750.0 g of water? (Kb = 0.512 C/m for water)
A)98.22 C
B)101.00 C
C)99.00 C
D)101.78 C
E)100.26 C
A)98.22 C
B)101.00 C
C)99.00 C
D)101.78 C
E)100.26 C

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
69
The vapor pressure of an aqueous solution is found to be 24.9 mmHg at 25 C.What is the mole fraction of solute in this solution? The vapor pressure of water is 25.756 mmHg at 25 C.
A)0.967
B)0.033
C)1.03
D)0.034
E)0.976
A)0.967
B)0.033
C)1.03
D)0.034
E)0.976

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following regarding the vapor pressure of a solution containing a nonvolatile solute is NOT correct?
A)The amount by which the vapor pressure of the solution is reduced reflects the mole fraction of the solute in the solution.
B)Raoult's law predicts that the solution containing a solute that experiences strong solute-solvent attractions will have a lower vapor pressure than an ideal solution.
C)The presence of a nonvolatile solute reduces the mole fraction of the pure solvent.
D)The evaporation rate of the pure solvent in an open container is higher than that of the solution.
E)The condensation rates of the pure solvent and the solution are probably about equal in a closed container.
A)The amount by which the vapor pressure of the solution is reduced reflects the mole fraction of the solute in the solution.
B)Raoult's law predicts that the solution containing a solute that experiences strong solute-solvent attractions will have a lower vapor pressure than an ideal solution.
C)The presence of a nonvolatile solute reduces the mole fraction of the pure solvent.
D)The evaporation rate of the pure solvent in an open container is higher than that of the solution.
E)The condensation rates of the pure solvent and the solution are probably about equal in a closed container.

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
71
You must mix 168 g of carbon disulfide (CS2, 76.13 g/mol) with acetonitrile (CH3CN, 41.06 g/mol) to create a solution that has a total vapor pressure of 292 torr at 25 C.Calculate the number of grams of CH3CN required. 
A)40.6 g
B)29.1 g
C)90.6 g
D)42.1 g
E)4.93 g

A)40.6 g
B)29.1 g
C)90.6 g
D)42.1 g
E)4.93 g

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
72
What is the boiling point elevation constant of ethanol if a solution prepared by dissolving 56.00 g of glycerin (C3H8O3, 92.11 g/mol) in 240.0 g of ethanol (C2H6O, 46.08 g/mol) has a boiling point change of 3.014 C?
A)1.029 C/m
B)0.6080 C/m
C)1.190 C/m
D)0.8405 C/m
E)0.4398 C/m
A)1.029 C/m
B)0.6080 C/m
C)1.190 C/m
D)0.8405 C/m
E)0.4398 C/m

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
73
The normal temperature range of the liquid phase of pure water is 0 C to 100 C.Which of the following solutions will have the largest temperature range for the liquid state?
A)1 M aqueous ethanol solution
B)1 M aqueous potassium bromide solution
C)1 M aqueous acetic acid solution
D)1 M aqueous magnesium bromide solution
E)1 M aqueous magnesium sulfate
A)1 M aqueous ethanol solution
B)1 M aqueous potassium bromide solution
C)1 M aqueous acetic acid solution
D)1 M aqueous magnesium bromide solution
E)1 M aqueous magnesium sulfate

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
74
A solution is prepared by mixing 3.50 mL of dichloromethane (CH2Cl2, 84.93 g/mol, 1.33 g/mL) with 3.50 mL of dibromomethane (CH2Br2, 173.8 g/mol, 2.48 g/mL).By what factor is the vapor phase enriched in CH2Cl2 at 25 C?

A)35.3
B)10.8
C)2.06
D)1.77
E)11.7

A)35.3
B)10.8
C)2.06
D)1.77
E)11.7

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
75
How many moles of solute are in a 0.174 m aspartic acid (C4H7NO4) solution prepared with 250.0 g of water?
A)6.96 *10-4 mol
B)4.35* 10-2 mol
C)0.696 mol
D)1.44 mol
E)43.5 mol
A)6.96 *10-4 mol
B)4.35* 10-2 mol
C)0.696 mol
D)1.44 mol
E)43.5 mol

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
76
A solution contains 6.50 mol of water, 0.300 mol of sucrose, and 0.200 mol of glucose.The solutes are nonvolatile.What is the vapor pressure of the solution at 35 C given that the vapor pressure of water is 42.2 torr?
A)35.0 torr
B)36.0 torr
C)37.0 torr
D)39.2 torr
E)39.0 torr
A)35.0 torr
B)36.0 torr
C)37.0 torr
D)39.2 torr
E)39.0 torr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
77
A solution is prepared by adding 0.300 mol of glucose, which is not volatile, to 4.50 mol of water.What is the vapor pressure of this solution at 25 C given that the vapor pressure of pure water is 23.8 torr?
A)9.38 torr
B)1.49 torr
C)23.4 torr
D)22.3 torr
E)22.5 torr
A)9.38 torr
B)1.49 torr
C)23.4 torr
D)22.3 torr
E)22.5 torr

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
78
Calculate the molality of a solution containing 0.755 mol of glucose C6H12O6) and 1750 g of water.
A)0.875 m
B)0.583 m
C)0.431 m
D)580 m
E)0.850 m
A)0.875 m
B)0.583 m
C)0.431 m
D)580 m
E)0.850 m

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
79
Determine the molal concentration of a table sugar (C12H22O11, 342.3 g/mol) solution in water that has a freezing point of -2.10 C.(Kf =1.86 C/m for water)
A)1.13 m
B)(-1.13 m)
C)3.91 m
D)(-3.91 m)
E)0.113 m
A)1.13 m
B)(-1.13 m)
C)3.91 m
D)(-3.91 m)
E)0.113 m

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck
80
What is the boiling point of a solution prepared by dissolving 10.00 g of naphthalene (C10H8, 128.17 g/mol) in 200.0 g of benzene (C6H6, 78.12 g/mol)? The normal boiling point of benzene is 80.1 C and Kb =2.53 C/m.
A)79.1 C
B)81.1 C
C)80.5 C
D)86.6 C
E)101.0 C
A)79.1 C
B)81.1 C
C)80.5 C
D)86.6 C
E)101.0 C

Unlock Deck
Unlock for access to all 130 flashcards in this deck.
Unlock Deck
k this deck


