Exam 11: Properties of Solutions Their Concentrations and Colligative Properties
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
Suppose 100.0 mL of a 2.50 mM NaCl solution was mixed with 100.0 mL of a 3.40 mM MgCl2 solution at 25 C.Assuming the volumes are additive and using the ideal van 't Hoff factors, what would be the osmotic pressure of the resulting solution?
Free
(Short Answer)
4.9/5  (30)
(30)
Correct Answer:
0.186 atm
A 50:50 mixture of cyclohexane (C6H12, 84.16 g/mol) and toluene (C6H5CH3, 92.14 C) are to be separated by fractional distillation.A plot of temperature versus volume of distillate shows two plateaus, one at 80.7 C and one at 110.6 C.What is the approximate boiling point of each liquid? Use intermolecular forces to justify your answer.
Free
(Essay)
4.9/5  (35)
(35)
Correct Answer:
The boiling point of C6H12 is probably 80.7 C.It has a lower molar mass, so the strength of the intermolecular forces should be lower.The boiling point of toluene, which is a larger molecule, is probably 110.6 C.
How many moles of solute are in a 0.174 m aspartic acid (C4H7NO4) solution prepared with 250.0 g of water?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
B
What is the molality of a lithium chloride solution produced by dissolving 14.40 g of LiCl (42.39 g/mol) in water to make 0.104 L of solution with a density of 1.102 g/mL?
(Multiple Choice)
4.9/5  (44)
(44)
What is the boiling point of a solution made by dissolving 25.0 g of powdered sulfur (S8, 256.52 g/mol) in 250.0 g of carbon disulfide (CS2, 76.13 g/mol, Kb = 2.37 C/m, Tb = 46.2 C)?
(Essay)
4.8/5  (32)
(32)
Which of the following regarding colligative properties of a solution is NOT correct?
(Multiple Choice)
4.9/5  (36)
(36)
The Henry's law constant for oxygen dissolving in blood is 3.74 * 10-2 mol/L . atm at body temperature, 37 C.Calculate the molar concentration of oxygen in blood for a scuba diver where the air pressure is 2.0 atm.The mole fraction of oxygen in air is 0.209.
(Multiple Choice)
4.8/5  (39)
(39)
What is the osmotic pressure of a 5.20% mass/volume aqueous ethanol (CH3CH2OH, 46.07 g/mol) solution at 25.0 C? Assume the density of the solution is 1.00 g/mL.
(Multiple Choice)
4.8/5  (36)
(36)
Two solutions, A and B, are separated by a semipermeable membrane.Which of the following statements regarding their tonicities is NOT correct? Assume the same van 't Hoff factor for each solution.
(Multiple Choice)
4.8/5  (33)
(33)
The Henry's law constant for oxygen dissolving in blood is 3.74 *10-2 mol/L . atm at body temperature, 37 C.Calculate the molar concentration of oxygen in blood for an alpine climber where the atmospheric pressure is 0.45 atm.The mole fraction of oxygen in air is 0.209.
(Multiple Choice)
4.8/5  (42)
(42)
A solution of 5.00 g of lithium chloride in 1.00 kg of water has a freezing point of -0.410 C.What is the actual van 't Hoff factor for this salt at this concentration? (Kf (water) =1.86 C/m)
(Short Answer)
4.8/5  (40)
(40)
A solution is prepared by mixing 75 g of methanol (CH3OH, 32.04 g/mol) with 25 g of ethanol (CH3CH2OH, 46.07 g/mol).What is the mole fraction of ethanol in the vapor phase at 20 C? 
(Multiple Choice)
4.8/5  (38)
(38)
A solution contains pentane (C5H12, 72.15 g/mol) and hexane (C6H14, 86.17 g/mol) at 25 C, which have vapor pressures of 511 and 150 torr, respectively.To create a solution with a total vapor pressure of 275 torr, approximately how many grams of hexane should be mixed with 50.0 g pentane?
(Short Answer)
4.7/5  (39)
(39)
Which of the following statements regarding the phase diagram of water and an aqueous solution is NOT correct? Temperature is on the x-axis; pressure is on the y-axis. 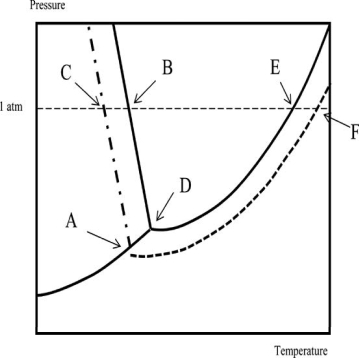
(Multiple Choice)
4.8/5  (46)
(46)
What is the osmotic pressure of a 0.0540 M aqueous sodium sulfate (Na2SO4, 142.04 g/mol) solution at 25.0 C?
(Multiple Choice)
5.0/5  (34)
(34)
Myristicin is a hallucinogenic compound found naturally in nutmeg.A 425 mg sample of myristicin was dissolved in 7.800 g of camphor (Kf = 39.7 C/m), decreasing the freezing point of camphor by 11.3 C.What is the molar mass of myristicin?
(Multiple Choice)
4.8/5  (40)
(40)
The normal boiling point of bromine is 58.8 C, and its enthalpy of vaporization is 30.91 kJ/mol.What is the approximate vapor pressure of bromine at 10.0 C?
(Multiple Choice)
4.8/5  (31)
(31)
Seawater can be characterized by the following average values.What is the molal concentration of ions in seawater? Explain how you arrived at your answer.
density = 1.022 g/mL,
total mass of ions = 35.17 g/kg
concentration of ions =1.15 M
(Essay)
4.8/5  (38)
(38)
A solution is prepared by mixing 3.50 mL of dichloromethane (CH2Cl2, 84.93 g/mol, 1.33 g/mL) with 3.50 mL of dibromomethane (CH2Br2, 173.8 g/mol, 2.48 g/mL).By what factor is the vapor phase enriched in CH2Cl2 at 25 C?

(Multiple Choice)
4.7/5  (35)
(35)
Showing 1 - 20 of 130
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)