Deck 16: Additional Aqueous Equilibria Chemistry and the Oceans
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/114
Play
Full screen (f)
Deck 16: Additional Aqueous Equilibria Chemistry and the Oceans
1
How many moles of sodium acetate must be added to 500.0 mL of 0.250 M acetic acid solution to produce a solution with a pH of 4.94? The pKa of acetic acid is 4.74.)
A)0.011 moles
B)0.021 moles
C)0.13 moles
D)0.20 moles
E)0.21 moles
A)0.011 moles
B)0.021 moles
C)0.13 moles
D)0.20 moles
E)0.21 moles
0.20 moles
2
What reaction occurs as sodium hydroxide is added to a solution containing equal concentrations of acetic acid and sodium acetate?
A)CH3 COOH(aq) + OH-aq) CH3 COO-(aq) + H2 O(l)
B)CH3 COO-(aq) +H2 Ol) CH3 COOH(aq) + OH-(aq)
C)CH3COO-(aq) + Na+(aq) NaCH3COO(aq)
D)2 CH3 COO-(aq) + 2 OH-(aq) 2 CH3 COOH(aq) + O2 (g)
E)CH3 COOH(aq) + Na+(aq) NaCH3 COO(aq) + H+(aq)
A)CH3 COOH(aq) + OH-aq) CH3 COO-(aq) + H2 O(l)
B)CH3 COO-(aq) +H2 Ol) CH3 COOH(aq) + OH-(aq)
C)CH3COO-(aq) + Na+(aq) NaCH3COO(aq)
D)2 CH3 COO-(aq) + 2 OH-(aq) 2 CH3 COOH(aq) + O2 (g)
E)CH3 COOH(aq) + Na+(aq) NaCH3 COO(aq) + H+(aq)
CH3 COOH(aq) + OH-aq) CH3 COO-(aq) + H2 O(l)
3
A solution that contains a weak acid and its conjugate base in roughly equal concentrations is
A)neither acidic or basic.
B)a half-acid solution.
C)a buffer.
D)a heterogeneous mixture.
E)neutral.
A)neither acidic or basic.
B)a half-acid solution.
C)a buffer.
D)a heterogeneous mixture.
E)neutral.
a buffer.
4
What is the pH of a solution prepared by dissolving 4.50 g sodium dihydrogen borate (83.82g/mol) in 650 mL of a 0.0725 M boric acid? The Ka of boric acid is 5.4 *10-10.Ignore any volume change.
A)9.21
B)9.32
C)9.27
D)9.40
E)9.14
A)9.21
B)9.32
C)9.27
D)9.40
E)9.14

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
5
Suppression of the solubility of one ion by the addition of the counter ion in its insoluble salt is known as the
A)ionic suppression effect.
B)counter-ion effect.
C)common-ion effect.
D)excession effect.
E)supersaturation effect.
A)ionic suppression effect.
B)counter-ion effect.
C)common-ion effect.
D)excession effect.
E)supersaturation effect.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
6
A solution containing roughly equal concentrations of is NOT a buffer solution.
A)fluoride ion and hydrofluoric acid
B)bromide ion and hydrobromic acid
C)phosphate ion and hydrogen phosphate ion
D)carbonate ion and hydrogen carbonate ion
E)phosphoric acid and dihydrogen phosphate ion
A)fluoride ion and hydrofluoric acid
B)bromide ion and hydrobromic acid
C)phosphate ion and hydrogen phosphate ion
D)carbonate ion and hydrogen carbonate ion
E)phosphoric acid and dihydrogen phosphate ion

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
7
A solution with a pH of 9.100 is prepared using aqueous ammonia and solid ammonium chloride.What is the ratio of [NH3] to [NH4+] in the solution? The Kb of ammonia is 1.76 * 10-5.
A)0.850 to 1
B)1.02 to 1
C)0.984 to 1
D)1.41 to 1
E)0.715 to 1
A)0.850 to 1
B)1.02 to 1
C)0.984 to 1
D)1.41 to 1
E)0.715 to 1

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
8
Consider the following aqueous equilibrium for a formic acid solution, HCOOH(aq) ⇄ H+(aq) + HCOO-(aq).Suppose solid sodium hydroxide is added to the solution.Which statement below regarding the resulting solution is FALSE? Ignore any volume changes.
A)The pH increases.
B)The H+ concentration decreases.
C)The system shifts right to reestablish equilibrium.
D)The concentration of HCOO- increases.
E)The value of Ka decreases.
A)The pH increases.
B)The H+ concentration decreases.
C)The system shifts right to reestablish equilibrium.
D)The concentration of HCOO- increases.
E)The value of Ka decreases.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
9
What is the pH of a solution in which [A-] = 2[HA] and the pKa of HA is 4.5?
A)2.5
B)4.2
C)4.5
D)4.8
E)6.5
A)2.5
B)4.2
C)4.5
D)4.8
E)6.5

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
10
A buffer solution is made by dissolving 0.230 moles of sodium hypochlorite in 1.00 L of 0.220 M hypochlorous acid.What is the pH of the solution after 0.0100 moles of sodium hydroxide is added? (The Ka of HClO is 2.9 * 10-8.)
A)6.520
B)6.404
C)7.578
D)7.480
E)7.596
A)6.520
B)6.404
C)7.578
D)7.480
E)7.596

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
11
What is the pH of a solution prepared by mixing 550.0 mL of 0.703 M CH3COOH with 460.0 mL of 0.905 M NaCH3COO? The Ka of acetic acid is 1.76 *10-5.Assume volumes are additive.
A)4.644
B)4.722
C)4.754
D)4.786
E)4.864
A)4.644
B)4.722
C)4.754
D)4.786
E)4.864

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
12
Which solution below would make the best buffer? (Ac = acetate)
A)hydrochloric acid and sodium chloride, HCl and NaCl
B)acetic acid and ammonia, HAc and NH3
C)acetic acid and ammonium chloride, HAc and NH4Cl
D)sodium acetate and ammonium chloride, NaAc and NH4Cl
E)ammonia and ammonium chloride, NH3 and NH4Cl
A)hydrochloric acid and sodium chloride, HCl and NaCl
B)acetic acid and ammonia, HAc and NH3
C)acetic acid and ammonium chloride, HAc and NH4Cl
D)sodium acetate and ammonium chloride, NaAc and NH4Cl
E)ammonia and ammonium chloride, NH3 and NH4Cl

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
13
Consider the following aqueous equilibrium for a formic acid solution, HCOOH(aq) ⇄ H+(aq) + HCOO-(aq).Suppose solid sodium formate, NaHCOO, is added to the solution.Which of the following statements regarding the resulting solution is NOT correct? Ignore any volume changes.
A)The pH decreases.
B)The H+ concentration decreases.
C)The system shifts left to reestablish equilibrium.
D)The HCOOH concentration increases.
E)The value of Ka does not change.
A)The pH decreases.
B)The H+ concentration decreases.
C)The system shifts left to reestablish equilibrium.
D)The HCOOH concentration increases.
E)The value of Ka does not change.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
14
Consider the following aqueous equilibrium for a formic acid solution, HCOOH(aq) ⇄ H+(aq) + HCOO-(aq).Suppose hydrogen chloride gas is dissolved in the solution.Which statement below regarding the resulting solution is FALSE? Ignore any volume changes.
A)The pH decreases.
B)The H+ concentration increases.
C)The system shifts left to reestablish equilibrium.
D)The concentration of HCOO- decreases.
E)The value of Ka increases.
A)The pH decreases.
B)The H+ concentration increases.
C)The system shifts left to reestablish equilibrium.
D)The concentration of HCOO- decreases.
E)The value of Ka increases.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
15
To simulate the pH of blood, which is 7.4, a buffer solution made by dissolving sodium dihydrogen phosphate (Ka = 6.2 *10-8) and sodium hydrogen phosphate (Ka= 3.6 *10-13) together in an aqueous solution can be used.What mole ratio of Na2HPO4/NaH2PO4 is required to produce a solution with a pH of 7.4?
A)1.2
B)1.6
C)0.90
D)1.0
E)0.96
A)1.2
B)1.6
C)0.90
D)1.0
E)0.96

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
16
What is the pH of a buffer solution in which [HA] = [A-]?
A)pH =1
B)pH = Ka
C)pH = pKa
D)pH = pOH
E)pH = 7.0
A)pH =1
B)pH = Ka
C)pH = pKa
D)pH = pOH
E)pH = 7.0

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the statements below about an acid-base buffer solution is/are true?
I.It can be prepared by combining a strong acid with a salt of its conjugate base.
II.It can be prepared by combining a weak acid with a salt of its conjugate base.
III.It can be prepared by combining a weak base with its conjugate acid.
IV.The pH of a buffer solution does not change when the solution is diluted.
V.A buffer solution resists changes in its pH when an acid or base is added to it.
A)I, II, and IV
B)II, III, and V
C)II, III, IV, and V
D)I, II, IV, and V
E)II, III, and IV
I.It can be prepared by combining a strong acid with a salt of its conjugate base.
II.It can be prepared by combining a weak acid with a salt of its conjugate base.
III.It can be prepared by combining a weak base with its conjugate acid.
IV.The pH of a buffer solution does not change when the solution is diluted.
V.A buffer solution resists changes in its pH when an acid or base is added to it.
A)I, II, and IV
B)II, III, and V
C)II, III, IV, and V
D)I, II, IV, and V
E)II, III, and IV

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
18
Research with biochemical systems commonly requires buffers because
A)it is less expensive to work at a constant pH.
B)proteins have a critical pH dependence in their structure and function.
C)proteins decompose into constituent amino acids outside a certain pH range.
D)proteins are buffers.
E)salts are involved.
A)it is less expensive to work at a constant pH.
B)proteins have a critical pH dependence in their structure and function.
C)proteins decompose into constituent amino acids outside a certain pH range.
D)proteins are buffers.
E)salts are involved.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
19
The pKa of a weak acid was determined by measuring the pH of a solution containing the weak acid at 0.40 M and its conjugate base at 0.60 M.The measured pH was 7.8.What is the pKa of the weak acid?
A)8.0
B)7.8
C)7.6
D)7.0
E)7.4
A)8.0
B)7.8
C)7.6
D)7.0
E)7.4

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
20
Which pairs of substances below can be mixed together in water to produce a buffer solution?
A)HClO4 and NaClO4
B)HNO3 and NaNO3
C)H2SO4 and NaHSO4
D)H3PO4 and NaH2PO4
E)HCl and NaCl
A)HClO4 and NaClO4
B)HNO3 and NaNO3
C)H2SO4 and NaHSO4
D)H3PO4 and NaH2PO4
E)HCl and NaCl

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
21
At the equivalence point of a strong acid-strong base titration, which species are typically present?
A)a weakly acidic cation, a weakly basic anion, and water
B)a weakly acidic cation, a non-basic anion, and water
C)a non-acidic cation, a weakly basic anion, and water
D)a non-acidic cation, a non-basic anion, and water
E)All of these are equally possible.
A)a weakly acidic cation, a weakly basic anion, and water
B)a weakly acidic cation, a non-basic anion, and water
C)a non-acidic cation, a weakly basic anion, and water
D)a non-acidic cation, a non-basic anion, and water
E)All of these are equally possible.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
22
The following titration curve is most likely to be associated with 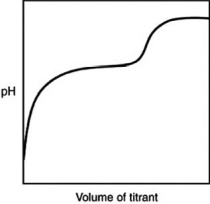
A)the titration of a strong acid with a strong base titrant.
B)the titration of a weak acid with a strong base titrant.
C)the titration of a strong base with a strong acid titrant.
D)the titration of a weak base with a strong acid titrant.

A)the titration of a strong acid with a strong base titrant.
B)the titration of a weak acid with a strong base titrant.
C)the titration of a strong base with a strong acid titrant.
D)the titration of a weak base with a strong acid titrant.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
23
In which of the following titrations would the solution be neutral at the equivalence point?
A)NH3 titrated with HCl
B)SrOH)2 titrated with H3PO4
C)HOCl titrated with BaOH)2
D)CH3COOH titrated with NaOH
E)HClO4 titrated with KOH
A)NH3 titrated with HCl
B)SrOH)2 titrated with H3PO4
C)HOCl titrated with BaOH)2
D)CH3COOH titrated with NaOH
E)HClO4 titrated with KOH

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
24
At what point in the following titration curve for a weak acid being titrated with a strong base is the pH equal to the pKa of the acid? The x-axis scale goes from 0.0 mL to 20.0 mL.The sharp rise is at 10.0 mL. 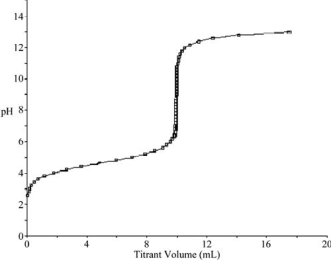
A)0.0 mL
B)5.0 mL
C)9.0 mL
D)10.0 mL
E)18.0 mL

A)0.0 mL
B)5.0 mL
C)9.0 mL
D)10.0 mL
E)18.0 mL

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
25
What is true about the pKa of the most suitable acid-base indicator for a titration of acetic acid with NaOH?
A)pK a of the indicator =pKa of the acid
B)pK a of the indicator =pKb of the base
C)pK a of the indicator = pH of a sodium acetate solution
D)pK a of the indicator = pH of an acetic acid solution
E)pK a of the indicator = 7.0
A)pK a of the indicator =pKa of the acid
B)pK a of the indicator =pKb of the base
C)pK a of the indicator = pH of a sodium acetate solution
D)pK a of the indicator = pH of an acetic acid solution
E)pK a of the indicator = 7.0

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
26
Acid-base indicators change color
A)exactly when pH = pKa of the indicator.
B)generally over a range of 1 or 2 pH units.
C)at pH = 7.
D)always between a pH of 6 and 8.
E)at the midpoint of a titration.
A)exactly when pH = pKa of the indicator.
B)generally over a range of 1 or 2 pH units.
C)at pH = 7.
D)always between a pH of 6 and 8.
E)at the midpoint of a titration.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
27
When an acetic acid solution is titrated with sodium hydroxide, the slope of the titration curve pH vs.volume of NaOH added) increases when sodium hydroxide is first added.This change shows that
A)nothing is happening during this part of the titration.
B)the reaction is very slow during this part of the titration.
C)a more concentrated solution of NaOH needs to be present to initiate the reaction.
D)acetic acid is being converted to sodium acetate.
E)the pH is not affected until all the acetic acid is consumed.
A)nothing is happening during this part of the titration.
B)the reaction is very slow during this part of the titration.
C)a more concentrated solution of NaOH needs to be present to initiate the reaction.
D)acetic acid is being converted to sodium acetate.
E)the pH is not affected until all the acetic acid is consumed.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
28
What is true about the pKa of the most suitable acid-base indicator for a titration of hydrochloric acid with NaOH?
A)pKa of the indicator =pKa of the acid
B)pKa of the indicator = pKb of the base
C)pK a of the indicator =the concentration of the acid
D)pK a of the indicator = the concentration of the base
E)pK a of the indicator =7.0
A)pKa of the indicator =pKa of the acid
B)pKa of the indicator = pKb of the base
C)pK a of the indicator =the concentration of the acid
D)pK a of the indicator = the concentration of the base
E)pK a of the indicator =7.0

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
29
Phenylephrine (PE; see the structure below) is a nasal decongestant and is the active ingredient in Sudafed, which contains phenylephrine hydrochloride (PEHCl).This conjugate acid of phenylephrine (PEH+) has a pKa= 5.5.At a physiological pH of 7.4, what is the ratio of concentrations, [PE]/[PEH+]? ![<strong>Phenylephrine (PE; see the structure below) is a nasal decongestant and is the active ingredient in Sudafed, which contains phenylephrine hydrochloride (PEHCl).This conjugate acid of phenylephrine (PEH<sup>+</sup>) has a pK<sub>a</sub>= 5.5.At a physiological pH of 7.4, what is the ratio of concentrations, [PE]/[PEH<sup>+</sup>]? </strong> A)6.7 B)0.01 C)0.14 D)79 E)21](https://storage.examlex.com/TB6562/11eb182e_a22b_9736_88c1_9d879d517106_TB6562_00.jpg)
A)6.7
B)0.01
C)0.14
D)79
E)21
![<strong>Phenylephrine (PE; see the structure below) is a nasal decongestant and is the active ingredient in Sudafed, which contains phenylephrine hydrochloride (PEHCl).This conjugate acid of phenylephrine (PEH<sup>+</sup>) has a pK<sub>a</sub>= 5.5.At a physiological pH of 7.4, what is the ratio of concentrations, [PE]/[PEH<sup>+</sup>]? </strong> A)6.7 B)0.01 C)0.14 D)79 E)21](https://storage.examlex.com/TB6562/11eb182e_a22b_9736_88c1_9d879d517106_TB6562_00.jpg)
A)6.7
B)0.01
C)0.14
D)79
E)21

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
30
Which solution below would be the best choice for preparing a buffer with a pH =5.0?
A)formic acid and sodium formate, Ka = 1.8 * 10-4
B)acetic acid and sodium acetate, Ka =1.8*10-5
C)hypochlorous acid and sodium hypochlorite, Ka =3.5*10-8
D)boric acid and sodium borate, Ka = 5.8 *10-10
E)All of these solutions would be equally good choices for making this buffer.
A)formic acid and sodium formate, Ka = 1.8 * 10-4
B)acetic acid and sodium acetate, Ka =1.8*10-5
C)hypochlorous acid and sodium hypochlorite, Ka =3.5*10-8
D)boric acid and sodium borate, Ka = 5.8 *10-10
E)All of these solutions would be equally good choices for making this buffer.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
31
Halfway to the equivalence point in a titration curve of a weak acid with a strong base,
A)nothing is happening yet.
B)pH = pKa of the weak acid.
C)pH = 3.5 exactly.
D)pH = pKa of the indicator.
E)the pH has not yet changed.
A)nothing is happening yet.
B)pH = pKa of the weak acid.
C)pH = 3.5 exactly.
D)pH = pKa of the indicator.
E)the pH has not yet changed.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
32
A buffer solution is made such that the initial concentrations of lactic acid (HC3H5O3 ) and the lactate ion (C3H5O3-) are 0.600 M and 0.620 M, respectively.What is the resulting pH if 100.0 mL of 0.100 M potassium hydroxide is added to 0.500 L of the buffer solution? (The Ka of HC3H5O3 is 1.4 * 10-4.)
A)3.81
B)3.90
C)2.75
D)4.96
E)3.85
A)3.81
B)3.90
C)2.75
D)4.96
E)3.85

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
33
Which combination of solutions is the best choice for making a buffer solution?
A)equal volumes of 1 M ammonia NH3) and 0.001 M ammonium chloride NH4Cl)
B)equal volumes of 0.5 M hydrochloric acid HCl) and 0.5 M sodium hydroxide NaOH)
C)equal volumes of 0.5 M hydrochloric acid HCl) and 0.5 M sodium chloride NaCl)
D)equal volumes of 2 M ammonia NH3) and 1 M hydrochloric acid HCl)
E)equal volumes of 2 M ammonium chloride NH4Cl) and 1 M hydrochloric acid HCl)
A)equal volumes of 1 M ammonia NH3) and 0.001 M ammonium chloride NH4Cl)
B)equal volumes of 0.5 M hydrochloric acid HCl) and 0.5 M sodium hydroxide NaOH)
C)equal volumes of 0.5 M hydrochloric acid HCl) and 0.5 M sodium chloride NaCl)
D)equal volumes of 2 M ammonia NH3) and 1 M hydrochloric acid HCl)
E)equal volumes of 2 M ammonium chloride NH4Cl) and 1 M hydrochloric acid HCl)

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
34
Acid-base indicators need to have very intense colors so that a very low concentration is visible.Could there be a problem in using too much indicator?
A)No, the colors would just be darker.
B)No, indicators are inert.
C)Yes, more indicator requires more extreme pH values to change color.
D)Yes, the indicator could affect the acid-base chemistry being measured.
E)No, the colors would just be sharper.
A)No, the colors would just be darker.
B)No, indicators are inert.
C)Yes, more indicator requires more extreme pH values to change color.
D)Yes, the indicator could affect the acid-base chemistry being measured.
E)No, the colors would just be sharper.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
35
What are the characteristics of a pH indicator? I.It is usually a conjugate acid-base pair.
II)It has different characteristic colors in protonated and unprotonated forms.
III)It changes color when the pH is near its pKa.
A)I only
B)II only
C)both II and III
D)I, II, and III
E)I and III
II)It has different characteristic colors in protonated and unprotonated forms.
III)It changes color when the pH is near its pKa.
A)I only
B)II only
C)both II and III
D)I, II, and III
E)I and III

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
36
Which solution below would be the best choice for preparing a buffer with a pH=8.0?
A)formic acid and sodium formate, Ka =1.8 * 10-4
B)acetic acid and sodium acetate, Ka = 1.8 *10-5
C)hypochlorous acid and sodium hypochlorite, Ka = 3.5 *10-8
D)boric acid and sodium borate, Ka =5.8 *10-10
E)All of these solutions would be equally good choices for making this buffer.
A)formic acid and sodium formate, Ka =1.8 * 10-4
B)acetic acid and sodium acetate, Ka = 1.8 *10-5
C)hypochlorous acid and sodium hypochlorite, Ka = 3.5 *10-8
D)boric acid and sodium borate, Ka =5.8 *10-10
E)All of these solutions would be equally good choices for making this buffer.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
37
A buffer solution is made such that the initial concentrations of lactic acid (HC3H5O3) and the lactate ion (C3H5O3-) are both 0.600 M.What is the resulting pH if 10.0 mL of 1.00 M hydrochloric acid is added to 0.500 L of the buffer solution? (The Ka of HC3H5O3 is 1.4 *10-4.)
A)3.82
B)3.88
C)2.92
D)4.79
E)3.85
A)3.82
B)3.88
C)2.92
D)4.79
E)3.85

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
38
Bromocresol green is yellow in its acidic form and blue in its basic form.When is it green?
A)at the equivalence point in a titration
B)in its neutral form
C)when the solution pH equals its pKa
D)when the solution pH equals 7
E)at the midpoint in a titration
A)at the equivalence point in a titration
B)in its neutral form
C)when the solution pH equals its pKa
D)when the solution pH equals 7
E)at the midpoint in a titration

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
39
A 25.00 mL sample of a hydrochloric acid solution was titrated to completion with 34.55 mL of 0.1020 M sodium hydroxide.What was the concentration of the hydrochloric acid?
A)0.07048 M
B)0.1410 M
C)0.2819 M
D)0.03524 M
E)0.05332 M
A)0.07048 M
B)0.1410 M
C)0.2819 M
D)0.03524 M
E)0.05332 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
40
In a titration of monoprotic acids and bases, there is a large change in pH
A)at the point where pH= pKa of the acid.
B)when the volume of acid is exactly equal to the volume of base.
C)when the concentration of acid is exactly equal to the concentration of base.
D)when the number of moles of acid is exactly equal to the number of moles of base.
E)at the point at which pH = pKb of the base.
A)at the point where pH= pKa of the acid.
B)when the volume of acid is exactly equal to the volume of base.
C)when the concentration of acid is exactly equal to the concentration of base.
D)when the number of moles of acid is exactly equal to the number of moles of base.
E)at the point at which pH = pKb of the base.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
41
The following titration curve is most likely to be associated with 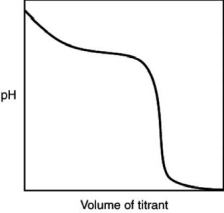
A)the titration of a strong acid with a strong base titrant.
B)the titration of a weak acid with a strong base titrant.
C)the titration of a strong base with a strong acid titrant.
D)the titration of a weak base with a strong acid titrant.

A)the titration of a strong acid with a strong base titrant.
B)the titration of a weak acid with a strong base titrant.
C)the titration of a strong base with a strong acid titrant.
D)the titration of a weak base with a strong acid titrant.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
42
Vitamin C is a monoprotic weak acid, which is also called ascorbic acid C6H8O5, 176.12 g/mol).A vitamin C tablet weighing 0.75 g was dissolved in 50.0 mL of water and titrated with 0.250 M sodium hydroxide.It took 12.5 mL of the NaOH solution to reach the endpoint.What is the percentage of vitamin C in the tablet? Only the vitamin C reacted with the NaOH.
A)98.7%
B)64.2%
C)87.7%
D)95.5%
E)73.3%
A)98.7%
B)64.2%
C)87.7%
D)95.5%
E)73.3%

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
43
What is indicated by the shape of the titration curve? 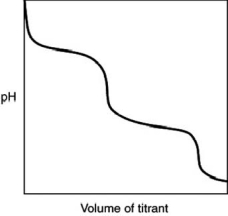
A)A diprotic acid was titrated with a strong base.
B)A triprotic acid was titrated with a strong base.
C)A dibasic base was titrated with a strong acid.
D)A tribasic base was titrated with a strong acid.
E)A strong acid was titrated with a strong base.

A)A diprotic acid was titrated with a strong base.
B)A triprotic acid was titrated with a strong base.
C)A dibasic base was titrated with a strong acid.
D)A tribasic base was titrated with a strong acid.
E)A strong acid was titrated with a strong base.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
44
A 25.00 mL sample of a phosphoric acid solution was titrated to completion with 37.04 mL of 0.1107 M sodium hydroxide.What was the concentration of the phosphoric acid?
A)0.05467 M
B)0.08201 M
C)0.1640 M
D)0.3280 M
E)0.4920 M
A)0.05467 M
B)0.08201 M
C)0.1640 M
D)0.3280 M
E)0.4920 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
45
Suppose a 1.0 L solution containing 0.20 moles of propanoic acid is titrated with 0.40 M KOH.The pH at the equivalence point is 8.99.What is the Ka of propanoic acid?
A)1.4 *10 -5
B)2.1 * 10 -5
C)5.1 *10 -9
D)7.2*10 -9
E)Insufficient information is provided to determine Ka.
A)1.4 *10 -5
B)2.1 * 10 -5
C)5.1 *10 -9
D)7.2*10 -9
E)Insufficient information is provided to determine Ka.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
46
Suppose a 1.0 L solution containing 0.20 moles of morphine is titrated with 0.40 M HCl.The pH at the equivalence point is 4.543.What is the Kb of morphine?
A)1.1*10 -6
B)9.4 -10 -9
C)1.6 - 10 -6
D)6.2 *10 -9
E)Insufficient information is provided to determine Kb.
A)1.1*10 -6
B)9.4 -10 -9
C)1.6 - 10 -6
D)6.2 *10 -9
E)Insufficient information is provided to determine Kb.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
47
A Lewis acid is
A)a proton donor.
B)a proton acceptor.
C)an electron-pair donor.
D)an electron-pair acceptor.
E)never viewed also as a Brønsted-Lowry acid.
A)a proton donor.
B)a proton acceptor.
C)an electron-pair donor.
D)an electron-pair acceptor.
E)never viewed also as a Brønsted-Lowry acid.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
48
Glycolic acid, which is a monoprotic acid and a constituent in sugarcane, has a pKa of 3.9.A 25.0 mL solution of glycolic acid is titrated to the equivalence point with 35.8 mL of 0.020 M sodium hydroxide solution.What is the pH of the resulting solution at the equivalence point?
A)4.12
B)9.88
C)6.01
D)7.99
E)Insufficient information is provided to determine the pH.
A)4.12
B)9.88
C)6.01
D)7.99
E)Insufficient information is provided to determine the pH.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
49
A Lewis base is any species capable of an electron pair.
A)accepting
B)donating
C)creating
D)neutralizing
E)gaining
A)accepting
B)donating
C)creating
D)neutralizing
E)gaining

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
50
One brand of extra-strength antacid tablets contains 750 mg of calcium carbonate 100.09 g/mol) in each tablet.Stomach acid is essentially a hydrochloric acid solution.Is so much calcium carbonate really needed to neutralize stomach acid? Calculate the volume of stomach acid with a pH of 1.00 that one of these tablets could neutralize, and compare that value with the normal volume of stomach fluid, which usually is about 100.0 mL.One tablet can neutralize mL of stomach acid at a pH of 1.00.
A)75
B)150
C)250
D)15
E)7.5
A)75
B)150
C)250
D)15
E)7.5

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
51
Identify the Lewis base in the following reaction: PH3 (aq) +H+(aq) PH4+ (aq)
A)PH4+
B)H+
C)PH3
D)None of these is a base.
E)All of these are bases.
A)PH4+
B)H+
C)PH3
D)None of these is a base.
E)All of these are bases.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
52
Identify the Lewis acid in the following reaction: PH3 (aq) +H+(aq) PH4+ (aq)
A)PH4+
B)H+
C)PH3
D)None of these is an acid.
E)All of these are acids.
A)PH4+
B)H+
C)PH3
D)None of these is an acid.
E)All of these are acids.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
53
A phosphate buffer solution 25.00 mL sample) used for a growth medium was titrated with 0.1000 M hydrochloric acid.The components of the buffer were sodium monohydrogenphosphate and sodium dihydrogenphosphate.The first endpoint occurred at a volume of 10.32 mL, and the second occurred after an additional 18.62 mL was added, for a total volume of 28.94 mL.What was the total concentration of phosphate in any form) in the buffer?
A)0.03992 M
B)0.1198 M
C)0.04243 M
D)0.1157 M
E)0.08382 M
A)0.03992 M
B)0.1198 M
C)0.04243 M
D)0.1157 M
E)0.08382 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
54
A 25.0 mL solution of quinine was titrated with 1.00 M hydrochloric acid, HCl.It was found that the solution contained 0.125 moles of quinine.What was the pH of the solution after 50.00 mL of the HCl solution were added? Quinine is monobasic with pKb = 5.10.
A)5.10
B)8.90
C)8.72
D)4.92
E)9.08
A)5.10
B)8.90
C)8.72
D)4.92
E)9.08

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
55
A 0.500 g sample of an unknown substance was titrated with a 0.1 M HCl solution.Another 0.500 g sample of it was titrated with a 0.1 M NaOH solution.The resulting titration curves are illustrated here.Given the following possibilities, what is the sample? 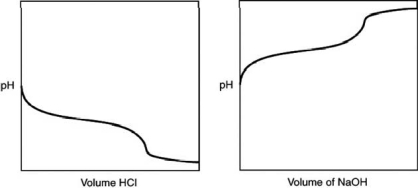
A)Na2CO3
B)NaHCO3
C)H2CO3
D)CO2
E)There is no way to tell.

A)Na2CO3
B)NaHCO3
C)H2CO3
D)CO2
E)There is no way to tell.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
56
A Lewis base is
A)an electron-pair acceptor.
B)an electron-pair donor.
C)a proton donor.
D)a proton acceptor.
E)never viewed also as a Brønsted-Lowry base.
A)an electron-pair acceptor.
B)an electron-pair donor.
C)a proton donor.
D)a proton acceptor.
E)never viewed also as a Brønsted-Lowry base.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
57
A solution of the weak acid, HF, and a solution of the strong acid, HCl, have the same pH.Which solution will require the most sodium hydroxide, NaOH, to neutralize?
A)HCl, because it is a strong acid and dissociates completely
B)HF, because its concentration is larger
C)Both will require the same amount because the concentrations are equal.
D)Both will require the same amount because the H3O+ concentrations are the same.
E)HCl, because the stronger acid has the higher concentration
A)HCl, because it is a strong acid and dissociates completely
B)HF, because its concentration is larger
C)Both will require the same amount because the concentrations are equal.
D)Both will require the same amount because the H3O+ concentrations are the same.
E)HCl, because the stronger acid has the higher concentration

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
58
A Lewis acid is any species capable of an electron pair.
A)accepting
B)donating
C)creating
D)neutralizing
E)losing
A)accepting
B)donating
C)creating
D)neutralizing
E)losing

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
59
Identify the Lewis base in the following reaction: SO2 (g) +H2 O(l) H2 SO3 (aq)
A)SO2
B)H2O
C)H2SO3
D)None of these is a base.
E)All of these are bases.
A)SO2
B)H2O
C)H2SO3
D)None of these is a base.
E)All of these are bases.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
60
A 200.0 mL solution of 0.40 M ammonium chloride was titrated with 0.80M sodium hydroxide.What was the pH of the solution after 50.0 mL of the NaOH solution were added? The Kb of ammonia is 1.76 *10-5.
A)9.25
B)4.75
C)11.22
D)5.02
E)8.98
A)9.25
B)4.75
C)11.22
D)5.02
E)8.98

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
61
What is the equilibrium concentration of Cl - (aq) in a solution that is initially 0.0200 M solution in CuNO3 and 0.450 M HCl(aq)?
Cu+(aq) + 3 Cl - 0(aq) fi CuCl32 - (aq) Kf = 5.0 * 105
A)0.450 M
B)2.0 * 10 - 6 M
C)0.390 M
D)0.430 M
E)5 * 105 M
Cu+(aq) + 3 Cl - 0(aq) fi CuCl32 - (aq) Kf = 5.0 * 105
A)0.450 M
B)2.0 * 10 - 6 M
C)0.390 M
D)0.430 M
E)5 * 105 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the statements below regarding hydrated metal ions is FALSE?
A)An aqueous metal ion can attract the electrons in the O-H bonds of the water molecules in its inner coordination sphere.
B)Aqueous metal ions catalyze the autoionization of water, causing the solution to become more acidic.
C)In solutions that are slightly acidic to slightly basic, most aqueous transition metal ions exist as complexes in which at least one ligand is OH-.
D)Acid strength typically increases as the charge on the aqueous metal ion increases.
E)Acid strength typically increases as the size of the aqueous metal ion decreases.
A)An aqueous metal ion can attract the electrons in the O-H bonds of the water molecules in its inner coordination sphere.
B)Aqueous metal ions catalyze the autoionization of water, causing the solution to become more acidic.
C)In solutions that are slightly acidic to slightly basic, most aqueous transition metal ions exist as complexes in which at least one ligand is OH-.
D)Acid strength typically increases as the charge on the aqueous metal ion increases.
E)Acid strength typically increases as the size of the aqueous metal ion decreases.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
63
What will be the equilibrium concentration of silver(I) ion in an aqueous solution originally containing 0.00100 M Ag+ and 0.500 M 1,10-phenanthroline?
Ag+(aq) + 2 phen(aq) fi [Ag(phen)2 ]1+(aq) Kf = 1.2=1012![<strong>What will be the equilibrium concentration of silver(I) ion in an aqueous solution originally containing 0.00100 M Ag<sup>+</sup> and 0.500 M 1,10-phenanthroline? Ag<sup>+</sup>(aq) + 2 phen(aq) fi [Ag(phen)<sub>2</sub> ]1<sup>+</sup>(aq) \quad K<sub>f</sub> = 1.2=10<sup>12</sup> </strong> A)9.0 *10<sup>-7</sup> M B)8.0 *10<sup>-13</sup> M C)6.0 *10<sup>-6</sup> M D)3.4 * 10<sup>-15</sup> M E)1.2*10<sup>-12</sup> M](https://storage.examlex.com/TB6562/11ed0816_5528_2398_91e4_cf5ed5e3ff68_TB6562_00.jpg)
A)9.0 *10-7 M
B)8.0 *10-13 M
C)6.0 *10-6 M
D)3.4 * 10-15 M
E)1.2*10-12 M
Ag+(aq) + 2 phen(aq) fi [Ag(phen)2 ]1+(aq) Kf = 1.2=1012
![<strong>What will be the equilibrium concentration of silver(I) ion in an aqueous solution originally containing 0.00100 M Ag<sup>+</sup> and 0.500 M 1,10-phenanthroline? Ag<sup>+</sup>(aq) + 2 phen(aq) fi [Ag(phen)<sub>2</sub> ]1<sup>+</sup>(aq) \quad K<sub>f</sub> = 1.2=10<sup>12</sup> </strong> A)9.0 *10<sup>-7</sup> M B)8.0 *10<sup>-13</sup> M C)6.0 *10<sup>-6</sup> M D)3.4 * 10<sup>-15</sup> M E)1.2*10<sup>-12</sup> M](https://storage.examlex.com/TB6562/11ed0816_5528_2398_91e4_cf5ed5e3ff68_TB6562_00.jpg)
A)9.0 *10-7 M
B)8.0 *10-13 M
C)6.0 *10-6 M
D)3.4 * 10-15 M
E)1.2*10-12 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following metal hydroxides is most likely to be more soluble in strongly basic solutions rather than weakly basic solutions?
A)MgOH)2
B)PbOH)2
C)CdOH)2
D)AlOH)3
E)FeOH)3
A)MgOH)2
B)PbOH)2
C)CdOH)2
D)AlOH)3
E)FeOH)3

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
65
What is the pH of a 0.65 M (CuNO3)2 solution?
(Ka for Cu2+(aq) = 3.0 *10-8
A)3.33
B)3.85
C)6.29
D)7.71
E)10.15
(Ka for Cu2+(aq) = 3.0 *10-8
A)3.33
B)3.85
C)6.29
D)7.71
E)10.15

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
66
What is the molar concentration of Ag+(aq) in a 1.00 M solution of Ag(NH3)2+ with no excess ammonia? (Kf = 1.70 * 107 for Ag(NH3)2+)
A)2.45 *10-3 M
B)3.09 * 10-3 M
C)2.42 * 10-4 M
D)1.47* 10-8 M
E)1.70 * 10-8 M
A)2.45 *10-3 M
B)3.09 * 10-3 M
C)2.42 * 10-4 M
D)1.47* 10-8 M
E)1.70 * 10-8 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
67
For the reaction Fe(H2O)63+(aq) +H2O(l) ⇄ Fe(H2O)5(OH)2+(aq) +H3O+(aq), Fe(H2O)63+(aq) is a(n) _______ and water is a(n) _______.
A)base; acid
B)acid; base
C)acid; catalyst
D)catalyst; base
E)Lewis base; Lewis acid
A)base; acid
B)acid; base
C)acid; catalyst
D)catalyst; base
E)Lewis base; Lewis acid

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
68
What is the equilibrium concentration of Cu+(aq) in a solution that is initially 0.0200 M solution in CuNO3 and 0.450 M HCl(aq)?
Cu+(aq) + 3 Cl-(aq) fi CuCl 2-(aq) Kf * 5.0 *105
A)5.0 F* 105 M
B)1.0 * 10-7 M
C)4.4 * 10-7 M
D)5.0 * 10-7 M
E)6.7 * 10-7 M
Cu+(aq) + 3 Cl-(aq) fi CuCl 2-(aq) Kf * 5.0 *105
A)5.0 F* 105 M
B)1.0 * 10-7 M
C)4.4 * 10-7 M
D)5.0 * 10-7 M

E)6.7 * 10-7 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
69
A coordinate covalent bond forms when a chemical species
A)donates one electron to another species to form a covalent bond.
B)accepts a pair of electrons and becomes an anion.
C)accepts one electron from another species to form a covalent bond.
D)accepts an electron and becomes an anion.
E)donates a pair of electrons to another species to form a covalent bond.
A)donates one electron to another species to form a covalent bond.
B)accepts a pair of electrons and becomes an anion.
C)accepts one electron from another species to form a covalent bond.
D)accepts an electron and becomes an anion.
E)donates a pair of electrons to another species to form a covalent bond.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
70
Calculate the equilibrium concentration of ZN2 + (aq) in a solution that is initially 0.0125 M Zn(NO3)2 and 0.600 M NH3.
Zn2+(aq) + 4 NH (aq) + Zn(NH3)4 2+(aq) Kf = 2.9 * 109
A)7.8 * 10-12 M
B)4.7 * 10- M
C)1.5 *10 - 8 M
D)2.5 * 10 - 9 M
E)2.9 * 109 M
Zn2+(aq) + 4 NH (aq) + Zn(NH3)4 2+(aq) Kf = 2.9 * 109
A)7.8 * 10-12 M
B)4.7 * 10- M
C)1.5 *10 - 8 M
D)2.5 * 10 - 9 M
E)2.9 * 109 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
71
What is the equilibrium concentration of ZN2+(aq) in a solution that is initially 0.0100 M solution in Zn(NO3)2 at a pH of 13.00?
Zn2+(aq) + 4 OH - (aq) fi Zn(OH)42 - (aq) Kf=
A)3.1 * 10-13 M
B)2.5 * 10-14 M
C)4.4 * 10-17 M
D)6.1* 10-10 M
E)5.0 * 105 M
Zn2+(aq) + 4 OH - (aq) fi Zn(OH)42 - (aq) Kf=
A)3.1 * 10-13 M
B)2.5 * 10-14 M
C)4.4 * 10-17 M
D)6.1* 10-10 M
E)5.0 * 105 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
72
A ligand is any forming a coordinate bond to a metal cation.
A)Lewis acid
B)ion
C)Lewis base
D)organic compound
E)species
A)Lewis acid
B)ion
C)Lewis base
D)organic compound
E)species

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
73
What is the solubility of barium sulfate in a solution containing 0.050 M sodium sulfate? The Ksp value for barium sulfate is 1.1 *10-10.
A)7.4 *10-6 M
B)5.5 *10-11 M
C)1.0 * 10-5 M
D)2.2 *10-9 M
E)1.1 * 10-10 M
A)7.4 *10-6 M
B)5.5 *10-11 M
C)1.0 * 10-5 M
D)2.2 *10-9 M
E)1.1 * 10-10 M

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
74
When sodium chloride is added to a saturated solution of leadII) chloride, some of the leadII) chloride precipitates.This phenomenon is called
A)the common-ion effect.
B)selective precipitation.
C)supersaturation.
D)a solubility anomaly.
E)deionization.
A)the common-ion effect.
B)selective precipitation.
C)supersaturation.
D)a solubility anomaly.
E)deionization.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
75
When NF3 reacts with BF3 to form F3NBF3,
A)a complex ion is formed.
B)an ionic bond is formed.
C)the bond is formed by sharing an electron from NF3 and an electron from BF3.
D)a coordinate covalent bond is formed.
E)BF3 donates an electron pair to NF3.
A)a complex ion is formed.
B)an ionic bond is formed.
C)the bond is formed by sharing an electron from NF3 and an electron from BF3.
D)a coordinate covalent bond is formed.
E)BF3 donates an electron pair to NF3.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
76
The solubility product for CaCO3 is written as _______, where s is the molar solubility.
A)Ksp = s2
B)Ksp = 3s2
C)Ksp = S3
D)Ksp = 9s4
E)Ksp= 27s4
A)Ksp = s2
B)Ksp = 3s2
C)Ksp = S3
D)Ksp = 9s4
E)Ksp= 27s4

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
77
Which statement below regarding complex ions and coordination compounds is FALSE?
A)Ligands function as electron pair donors.
B)A metal cation acts as a Lewis acid in a complex ion.
C)Ligands occupy the inner coordination sphere of the cation.
D)The information constant Kf for complex ions are generally much greater than 1.
E)Counter ions form coordinate covalent bonds with the ligands in a complex.
A)Ligands function as electron pair donors.
B)A metal cation acts as a Lewis acid in a complex ion.
C)Ligands occupy the inner coordination sphere of the cation.
D)The information constant Kf for complex ions are generally much greater than 1.
E)Counter ions form coordinate covalent bonds with the ligands in a complex.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following species is a Lewis base?
A)H+
B)Cs+
C)NF3
D)NF4+
E)NH4+
A)H+
B)Cs+
C)NF3
D)NF4+
E)NH4+

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following salts, when dissolved in pure water, causes the greatest pH change?
A)BaCl2
B)NaCl
C)CuCl2
D)FeCl3
E)ZnCl2
A)BaCl2
B)NaCl
C)CuCl2
D)FeCl3
E)ZnCl2

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck
80
Hydrated transition metal ions typically produce solutions that are
A)acidic.
B)basic.
C)neutral.
D)strongly basic.
E)strongly acidic.
A)acidic.
B)basic.
C)neutral.
D)strongly basic.
E)strongly acidic.

Unlock Deck
Unlock for access to all 114 flashcards in this deck.
Unlock Deck
k this deck


