Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans
Exam 1: Matter and Energy an Atomic Perspective138 Questions
Exam 2: Atoms, Ions, and Molecules the Building Blocks of Matter143 Questions
Exam 3: Atomic Structure Explaining the Properties of Elements175 Questions
Exam 4: Chemical Bonding Understanding Climate Change182 Questions
Exam 5: Bonding Theories Explaining Molecular Geometry141 Questions
Exam 6: Intermolecular Forces Attractions Between Particles87 Questions
Exam 7: Stoichiometry Mass Relationships and Chemical Reactions140 Questions
Exam 8: Aqueous Solutions Chemistry of the Hydrosphere180 Questions
Exam 9: Thermochemistry Energy Changes in Chemical Reactions215 Questions
Exam 10: Properties of Gases the Air We Breathe164 Questions
Exam 11: Properties of Solutions Their Concentrations and Colligative Properties130 Questions
Exam 12: Thermodynamics Why Chemical Reactions Happen130 Questions
Exam 13: Chemical Kinetics Clearing the Air172 Questions
Exam 14: Chemical Equilibrium Equal but Opposite Reaction Rates119 Questions
Exam 15: Acid-Base Equilibria Proton Transfer in Biological Systems123 Questions
Exam 16: Additional Aqueous Equilibria Chemistry and the Oceans114 Questions
Exam 17: Electrochemistry the Quest for Clean Energy135 Questions
Exam 18: The Solid State a Particulate View170 Questions
Exam 19: Organic Chemistry Fuels, Pharmaceuticals, and Modern Materials145 Questions
Exam 20: Biochemistry the Compounds of Life153 Questions
Exam 21: Nuclear Chemistry the Risks and Benefits168 Questions
Exam 22: The Main Group Elements Life and the Periodic Table116 Questions
Exam 23: Transition Metals Biological and Medical Applications119 Questions
Select questions type
Consider the following aqueous equilibrium for a formic acid solution, HCOOH(aq) ⇄ H+(aq) + HCOO-(aq).Suppose hydrogen chloride gas is dissolved in the solution.Which statement below regarding the resulting solution is FALSE? Ignore any volume changes.
Free
(Multiple Choice)
4.8/5  (46)
(46)
Correct Answer:
E
Halfway to the equivalence point in a titration curve of a weak acid with a strong base,
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
B
What is the solubility of barium sulfate in a solution containing 0.050 M sodium sulfate? The Ksp value for barium sulfate is 1.1 *10-10.
(Multiple Choice)
4.8/5  (35)
(35)
What is the pH of a 0.65 M (CuNO3)2 solution?
(Ka for Cu2+(aq) = 3.0 *10-8
(Multiple Choice)
4.9/5  (34)
(34)
A 25.0 mL solution of quinine was titrated with 1.00 M hydrochloric acid, HCl.It was found that the solution contained 0.125 moles of quinine.What was the pH of the solution after 50.00 mL of the HCl solution were added? Quinine is monobasic with pKb = 5.10.
(Multiple Choice)
4.9/5  (42)
(42)
Describe the similarities and differences between a covalent bond and a coordinate covalent bond.
(Essay)
5.0/5  (49)
(49)
A solution that contains a weak acid and its conjugate base in roughly equal concentrations is
(Multiple Choice)
4.7/5  (37)
(37)
If 500 mL of a solution containing 1.00 g of barium nitrate (199.33 g/mol) is combined with 500 mL of a solution containing 1.00 g of sodium sulfate (142.03 g/mol), will there be a precipitate? (Barium sulfate has a Ksp value of 9.1 * 10-11.)
(Multiple Choice)
4.8/5  (37)
(37)
What is the molar concentration of Ag+(aq) in a 1.00 M solution of Ag(NH3)2+ with no excess ammonia? (Kf = 1.70 * 107 for Ag(NH3)2+)
(Multiple Choice)
4.8/5  (47)
(47)
Which of the following compounds does NOT have a pH-dependent solubility?
(Multiple Choice)
4.8/5  (38)
(38)
At the equivalence point of a strong acid-strong base titration, which species are typically present?
(Multiple Choice)
4.8/5  (35)
(35)
What is the pH of a solution prepared by mixing 550.0 mL of 0.703 M CH3COOH with 460.0 mL of 0.905 M NaCH3COO? The Ka of acetic acid is 1.76 *10-5.Assume volumes are additive.
(Multiple Choice)
4.8/5  (39)
(39)
The following titration curve is most likely to be associated with 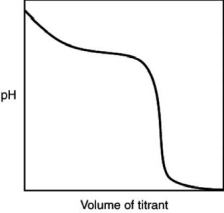
(Multiple Choice)
4.9/5  (32)
(32)
Suppose a 1.0 L solution containing 0.20 moles of propanoic acid is titrated with 0.40 M KOH.The pH at the equivalence point is 8.99.What is the Ka of propanoic acid?
(Multiple Choice)
4.7/5  (39)
(39)
Calculate the equilibrium concentration of ZN2 + (aq) in a solution that is initially 0.0125 M Zn(NO3)2 and 0.600 M NH3.
Zn2+(aq) + 4 NH (aq) + Zn(NH3)4 2+(aq) Kf = 2.9 * 109
(Multiple Choice)
5.0/5  (41)
(41)
Vitamin C is a monoprotic weak acid, which is also called ascorbic acid (C6H8O5, 176.12 g/mol).A vitamin C tablet weighing 0.75 g was dissolved in 50.0 mL of water and titrated with 0.250 M sodium hydroxide.It took 12.5 mL of the NaOH solution to reach the endpoint.What is the percentage of vitamin C in the tablet? Only the vitamin C reacted with the NaOH.
(Multiple Choice)
4.8/5  (33)
(33)
Chromium(III) hydroxide shows greater solubility in strongly basic aqueous solutions than in solutions that are weakly basic.Briefly explain why, and show any relevant reactions or equilibria.
(Essay)
4.8/5  (41)
(41)
The solubility product for CaCO3 is written as _______, where s is the molar solubility.
(Multiple Choice)
4.8/5  (36)
(36)
Showing 1 - 20 of 114
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)