Deck 2: Biochemistry Basics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 2: Biochemistry Basics
1
Isotopes are atoms of the same element that differ in
A) the number of neutrons found in the nucleus.
B) the number of protons and neutrons found in the nucleus.
C) the number of electrons orbiting the nucleus.
D) the number of protons and electrons found in the atom.
E) the number of protons found in the nucleus.
A) the number of neutrons found in the nucleus.
B) the number of protons and neutrons found in the nucleus.
C) the number of electrons orbiting the nucleus.
D) the number of protons and electrons found in the atom.
E) the number of protons found in the nucleus.
the number of neutrons found in the nucleus.
2
A feature of many of the isotopes that are used in the field of medicine is that the isotopes are radioactive. What does this mean?
A) Anionic forms of the atoms are used.
B) Cationic forms of the atom are used.
C) The atoms with the greatest atomic mass are used.
D) The nucleus of the isotope is unstable and breaks down over time.
E) The same number of atoms are arranged into different molecular structures.
A) Anionic forms of the atoms are used.
B) Cationic forms of the atom are used.
C) The atoms with the greatest atomic mass are used.
D) The nucleus of the isotope is unstable and breaks down over time.
E) The same number of atoms are arranged into different molecular structures.
The nucleus of the isotope is unstable and breaks down over time.
3
In the figure shown, what does the dotted line represent? 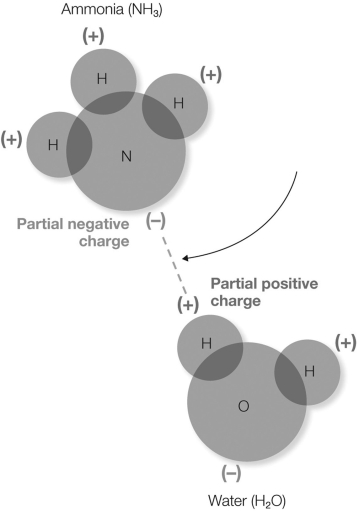
A) transfer of the electron from the hydrogen atom to the nitrogen atom
B) sharing of electrons between the ammonia and water molecules
C) a Van der Waals interaction
D) an electrostatic interaction between the partially- positive hydrogen and the partially- negative nitrogen
E) an interaction between a hydrophilic and a hydrophobic molecule

A) transfer of the electron from the hydrogen atom to the nitrogen atom
B) sharing of electrons between the ammonia and water molecules
C) a Van der Waals interaction
D) an electrostatic interaction between the partially- positive hydrogen and the partially- negative nitrogen
E) an interaction between a hydrophilic and a hydrophobic molecule
an electrostatic interaction between the partially- positive hydrogen and the partially- negative nitrogen
4
A compound which stabilizes pH by absorbing or releasing H+ ions is called a(n)
A) acid.
B) buffer.
C) salt.
D) base.
E) solute.
A) acid.
B) buffer.
C) salt.
D) base.
E) solute.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
In a polar covalent bond,
A) electrons are shared unequally between two atoms.
B) electrons are transferred from one atom to another.
C) an acid and a base neutralize each other to form a salt.
D) electrons are shared unequally between more than two atoms.
E) electrons are shared equally between two atoms.
A) electrons are shared unequally between two atoms.
B) electrons are transferred from one atom to another.
C) an acid and a base neutralize each other to form a salt.
D) electrons are shared unequally between more than two atoms.
E) electrons are shared equally between two atoms.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is incorrectly matched?
A) Base: Release hydroxide ions (OH- ) in an aqueous solution
B) pH: Measure of the acidity or basicity of a solution
C) Acid: Release hydrogen ions (H+) in an aqueous solution
D) Salt: Formed by the combination of hydrogen ions (H+) and hydroxide ions (OH- )
E) Water: Is the solvent in aqueous solutions
A) Base: Release hydroxide ions (OH- ) in an aqueous solution
B) pH: Measure of the acidity or basicity of a solution
C) Acid: Release hydrogen ions (H+) in an aqueous solution
D) Salt: Formed by the combination of hydrogen ions (H+) and hydroxide ions (OH- )
E) Water: Is the solvent in aqueous solutions

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
Compared to a solution with a pH value of 4, a solution with a pH value of 2 has H+ ions.
A) half as many
B) the same number of
C) one hundred times as many
D) one hundred times fewer
E) twice as many
A) half as many
B) the same number of
C) one hundred times as many
D) one hundred times fewer
E) twice as many

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
An atom is best described as
A) the smallest unit of an element.
B) defined by its number of electrons.
C) defined by its atomic mass.
D) always containing an equal number of protons and neutrons.
E) having a nucleus containing protons and electrons.
A) the smallest unit of an element.
B) defined by its number of electrons.
C) defined by its atomic mass.
D) always containing an equal number of protons and neutrons.
E) having a nucleus containing protons and electrons.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
The pictured molecules both contain six carbon atoms, twelve hydrogen atoms, and six oxygen atoms (C6H12O6). However, these atoms are arranged differently in each molecule. What are these molecules called? 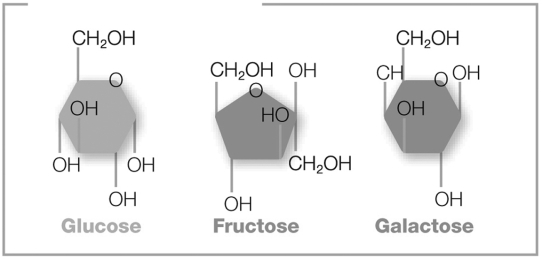
A) anions
B) R groups
C) functional groups
D) isomers
E) isotopes

A) anions
B) R groups
C) functional groups
D) isomers
E) isotopes

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following shows an ionic bond?
A)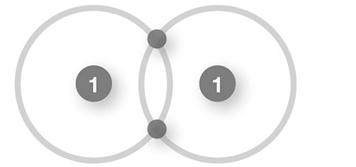
B)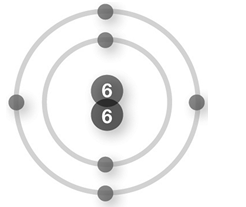
C)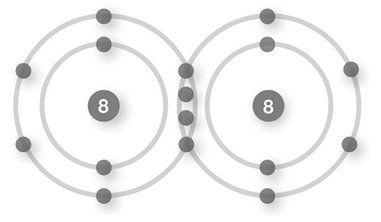
D)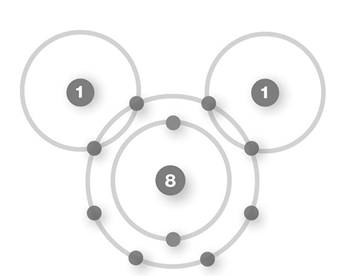
E)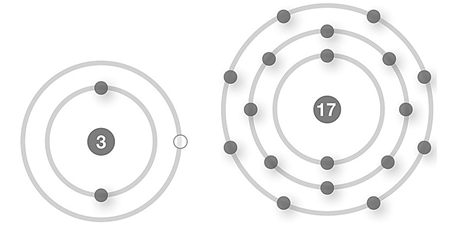
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
What information can you determine about the element nitrogen from the periodic table entry shown? 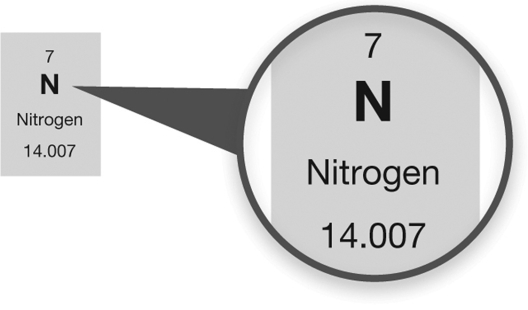
A) The atomic number for nitrogen is 7 and there are 14 neutrons in the nucleus of a nitrogen atom.
B) The atomic number for nitrogen is 14.007 and there are 14 neutrons in the nucleus of a nitrogen atom.
C) The atomic number for nitrogen is 7.
D) There are 14 neutrons in the nucleus of a nitrogen atom.
E) The atomic number for nitrogen is 14.007.

A) The atomic number for nitrogen is 7 and there are 14 neutrons in the nucleus of a nitrogen atom.
B) The atomic number for nitrogen is 14.007 and there are 14 neutrons in the nucleus of a nitrogen atom.
C) The atomic number for nitrogen is 7.
D) There are 14 neutrons in the nucleus of a nitrogen atom.
E) The atomic number for nitrogen is 14.007.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
Which particle is described incorrectly?
A) Neutron: Found in the nucleus and 1 atomic mass unit
B) Proton: Positively charged and 1 atomic mass unit
C) Electron: Negatively charged and negligible mass
D) Proton: Found in shells orbiting the nucleus
E) Electron: Found in shells orbiting the nucleus
A) Neutron: Found in the nucleus and 1 atomic mass unit
B) Proton: Positively charged and 1 atomic mass unit
C) Electron: Negatively charged and negligible mass
D) Proton: Found in shells orbiting the nucleus
E) Electron: Found in shells orbiting the nucleus

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Ionic bonds
A) form when electrons are transferred from atom to another.
B) are electrostatic forces of attraction between oppositely charged ions.
C) only exist as ions in solution.
D) form when electrons are transferred from atom to another and only exist as ions in solution.
E) are electrostatic forces of attraction between oppositely charged ions and form when electrons are transferred from atom to another.
A) form when electrons are transferred from atom to another.
B) are electrostatic forces of attraction between oppositely charged ions.
C) only exist as ions in solution.
D) form when electrons are transferred from atom to another and only exist as ions in solution.
E) are electrostatic forces of attraction between oppositely charged ions and form when electrons are transferred from atom to another.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
An anion is formed when
A) an atom loses one or more positively- charged protons.
B) an atom gains one or more negatively- charged electrons.
C) an atom loses one or more negatively- charged electrons.
D) an atom has an equal number of positively- charged protons and negatively- charged electrons.
E) an atom gains one or more positively- charged protons.
A) an atom loses one or more positively- charged protons.
B) an atom gains one or more negatively- charged electrons.
C) an atom loses one or more negatively- charged electrons.
D) an atom has an equal number of positively- charged protons and negatively- charged electrons.
E) an atom gains one or more positively- charged protons.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
Which statement is true about valence electrons?
A) Valence electrons participate in chemical reactions, and interactions between valence electrons in reacting atoms determine what kind of chemical bond is formed.
B) Valence electrons are found in the innermost shell.
C) Valence electrons are found in the innermost shell and participate in chemical reactions.
D) Valence electrons participate in chemical reactions.
E) Interactions between valence electrons in reacting atoms determine what kind of chemical bond is formed.
A) Valence electrons participate in chemical reactions, and interactions between valence electrons in reacting atoms determine what kind of chemical bond is formed.
B) Valence electrons are found in the innermost shell.
C) Valence electrons are found in the innermost shell and participate in chemical reactions.
D) Valence electrons participate in chemical reactions.
E) Interactions between valence electrons in reacting atoms determine what kind of chemical bond is formed.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
Electrolytes
A) are ionic compounds dissolved in solution.
B) include acids, bases, and salts.
C) include acids, bases, and salts, are ionic compounds dissolved in solution and are involved in regulating the nervous system, heartbeat, blood volume and water balance in the body.
D) are ionic compounds dissolved in solution and are involved in regulating the nervous system, heartbeat, blood volume and water balance in the body.
E) are involved in regulating the nervous system, heartbeat, blood volume and water balance in the body.
A) are ionic compounds dissolved in solution.
B) include acids, bases, and salts.
C) include acids, bases, and salts, are ionic compounds dissolved in solution and are involved in regulating the nervous system, heartbeat, blood volume and water balance in the body.
D) are ionic compounds dissolved in solution and are involved in regulating the nervous system, heartbeat, blood volume and water balance in the body.
E) are involved in regulating the nervous system, heartbeat, blood volume and water balance in the body.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
Which functional group is incorrectly matched with its structure?
A) ether: R- CH3
B) amino: R- NH2
C) phosphate: R- PO42-
D) carboxyl: R- COOH
E) alcohol: R- OH
A) ether: R- CH3
B) amino: R- NH2
C) phosphate: R- PO42-
D) carboxyl: R- COOH
E) alcohol: R- OH

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is an organic compound?
A) carbon dioxide (CO2)
B) methane (CH4)
C) ethanol (C2H6O)
D) carbon dioxide (CO2), ethanol (C2H6O), and methane (CH4)
E) ethanol (C2H6O) and methane (CH4)
A) carbon dioxide (CO2)
B) methane (CH4)
C) ethanol (C2H6O)
D) carbon dioxide (CO2), ethanol (C2H6O), and methane (CH4)
E) ethanol (C2H6O) and methane (CH4)

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
In the figure shown, which atom(s) will have a partial negative charge? 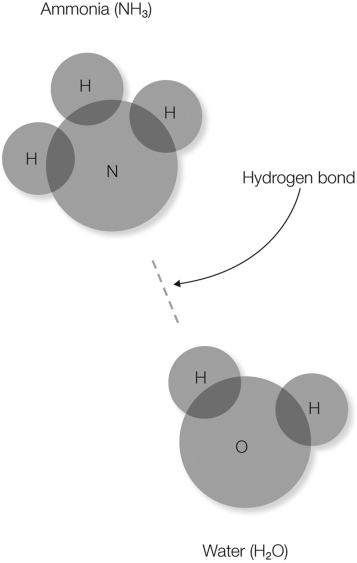
A) nitrogen and oxygen
B) hydrogen
C) hydrogen and oxygen
D) oxygen
E) nitrogen

A) nitrogen and oxygen
B) hydrogen
C) hydrogen and oxygen
D) oxygen
E) nitrogen

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
Pure water is defined by
A) a neutral pH of 7 and an equal number of H+ and OH- ions.
B) a neutral pH of 7.
C) a neutral pH of 7, and equal number of H+ and OH- ions, and its ability to serve as a pH buffer in solutions.
D) an equal number of H+ and OH- ions.
E) its ability to serve as a pH buffer in solutions.
A) a neutral pH of 7 and an equal number of H+ and OH- ions.
B) a neutral pH of 7.
C) a neutral pH of 7, and equal number of H+ and OH- ions, and its ability to serve as a pH buffer in solutions.
D) an equal number of H+ and OH- ions.
E) its ability to serve as a pH buffer in solutions.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the biomolecules is incorrectly matched with its building block?
A) Protein: Amino acid
B) Lipid: Fatty acid
C) Lipid: Glycerol
D) Nucleic acid: Nucleotide
E) Carbohydrate: Polysaccharide
A) Protein: Amino acid
B) Lipid: Fatty acid
C) Lipid: Glycerol
D) Nucleic acid: Nucleotide
E) Carbohydrate: Polysaccharide

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
What type of reaction does the figure show? 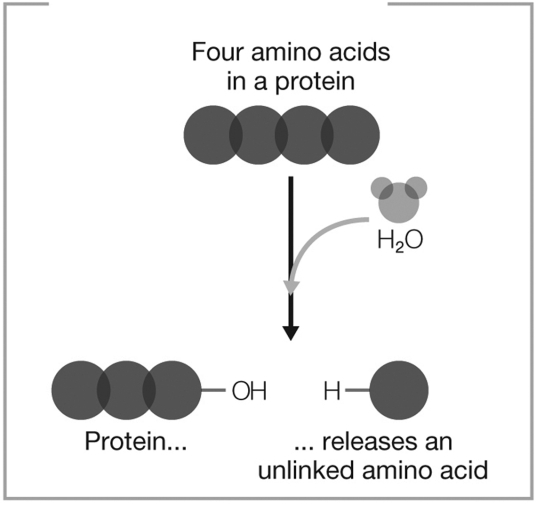
A) decomposition and hydrolysis
B) dehydration synthesis
C) dehydration synthesis and hydrolysis
D) decomposition
E) hydrolysis

A) decomposition and hydrolysis
B) dehydration synthesis
C) dehydration synthesis and hydrolysis
D) decomposition
E) hydrolysis

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
A micelle is formed of
A) both hydrophilic and hydrophobic molecules.
B) hydrophilic molecules only.
C) amphipathic molecules where the hydrophobic portion faces toward the center.
D) amphipathic molecules where the hydrophilic portion faces toward the center.
E) hydrophobic molecules only.
A) both hydrophilic and hydrophobic molecules.
B) hydrophilic molecules only.
C) amphipathic molecules where the hydrophobic portion faces toward the center.
D) amphipathic molecules where the hydrophilic portion faces toward the center.
E) hydrophobic molecules only.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
Van der Waals interactions
A) are responsible for the repulsion between hydrophilic and hydrophobic compounds.
B) exhibit a force of repulsion that serves to destabilize molecules.
C) are another name for hydrogen bonds.
D) occur when temporary dipoles within molecules form that are not the result of hydrogen bond to O, N, or F atoms.
E) are stronger than either hydrogen bonds or ionic bonds.
A) are responsible for the repulsion between hydrophilic and hydrophobic compounds.
B) exhibit a force of repulsion that serves to destabilize molecules.
C) are another name for hydrogen bonds.
D) occur when temporary dipoles within molecules form that are not the result of hydrogen bond to O, N, or F atoms.
E) are stronger than either hydrogen bonds or ionic bonds.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
Which statement does not describe a catalyst?
A) A catalyst is not used up in the reaction.
B) A catalyst will increase the rate of a reaction.
C) A catalyst may be an inorganic substance.
D) A catalyst may be an organic substance.
E) A catalyst will decrease the rate of a reaction.
A) A catalyst is not used up in the reaction.
B) A catalyst will increase the rate of a reaction.
C) A catalyst may be an inorganic substance.
D) A catalyst may be an organic substance.
E) A catalyst will decrease the rate of a reaction.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
The type of bond which links the amino group of one amino acid to the carboxyl group of another amino acid is called a(n)
A) glycosidic bond.
B) amino bond.
C) peptide bond.
D) glycerol bond.
E) phosphodiester bond.
A) glycosidic bond.
B) amino bond.
C) peptide bond.
D) glycerol bond.
E) phosphodiester bond.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
Deoxyribonucleotides and ribonucleotides differ in all of the following except
A) phosphodiester bonds can only form between deoxyribonucleotides, not ribonucleotides.
B) deoxyribonucleotides are found in DNA while ribonucleotides are found in RNA.
C) the sugar is different in the two types of nucleotides.
D) ribonucleotides can serve as energy molecules while deoxyribonucleotides do not.
E) the nitrogenous base thymine is only found in deoxyribonucleotides while the nitrogenous base uracil is only found in ribonucleotides.
A) phosphodiester bonds can only form between deoxyribonucleotides, not ribonucleotides.
B) deoxyribonucleotides are found in DNA while ribonucleotides are found in RNA.
C) the sugar is different in the two types of nucleotides.
D) ribonucleotides can serve as energy molecules while deoxyribonucleotides do not.
E) the nitrogenous base thymine is only found in deoxyribonucleotides while the nitrogenous base uracil is only found in ribonucleotides.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
Which term is incorrectly matched to its description?
A) Amphipathic: Has properties that are neither hydrophilic nor hydrophobic; hydrophilic: substances which readily dissolve in water; AND hydrophobic: substances which are not readily dissolved in water are all incorrectly matched
B) Hydrophobic: Substances which are not readily dissolved in water
C) Hydrophilic: Substances which readily dissolve in water
D) Amphipathic: Has properties that are neither hydrophilic nor hydrophobic
E) Hydrophilic: Substances which readily dissolve in water AND hydrophobic: substances which are not readily dissolved in water are both incorrectly matched.
A) Amphipathic: Has properties that are neither hydrophilic nor hydrophobic; hydrophilic: substances which readily dissolve in water; AND hydrophobic: substances which are not readily dissolved in water are all incorrectly matched
B) Hydrophobic: Substances which are not readily dissolved in water
C) Hydrophilic: Substances which readily dissolve in water
D) Amphipathic: Has properties that are neither hydrophilic nor hydrophobic
E) Hydrophilic: Substances which readily dissolve in water AND hydrophobic: substances which are not readily dissolved in water are both incorrectly matched.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
In a dehydration synthesis reaction, macromolecules are built when is removed to form a covalent bond.
A) an electron
B) carbon dioxide
C) water
D) an amino acid
E) oxygen
A) an electron
B) carbon dioxide
C) water
D) an amino acid
E) oxygen

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the reaction AB - A + B. What is the product of this reaction?
A) A
B) B
C) AB
D) A and B
E) A, B, and AB
A) A
B) B
C) AB
D) A and B
E) A, B, and AB

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following does not describe equilibrium?
A) A forward and reverse reaction occur at the same rate.
B) when there is an equal amount of products and reactants and a reaction has stopped
C) A reaction has stopped.
D) The total amount of products and reactants is no longer changing.
E) when there is an equal amount of products and reactants
A) A forward and reverse reaction occur at the same rate.
B) when there is an equal amount of products and reactants and a reaction has stopped
C) A reaction has stopped.
D) The total amount of products and reactants is no longer changing.
E) when there is an equal amount of products and reactants

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
The figure shown is an exergonic reaction because 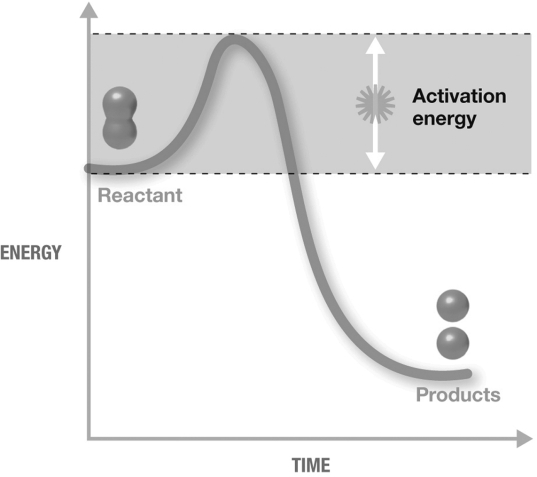
A) activation energy was required.
B) it is a decomposition reaction and the products have a lower final energy than the reactants.
C) it is a decomposition reaction.
D) the products have a lower final energy than the reactants.
E) activation energy was required and the products have a lower final energy than the reactants.

A) activation energy was required.
B) it is a decomposition reaction and the products have a lower final energy than the reactants.
C) it is a decomposition reaction.
D) the products have a lower final energy than the reactants.
E) activation energy was required and the products have a lower final energy than the reactants.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
Plasma membranes, the key boundary layer of cells, are composed of amphipathic molecules called phospholipids. Why would neither purely hydrophilic nor purely hydrophobic molecules be a suitable molecule for plasma membranes?
A) Hydrophilic molecules would dissolve in the aqueous environment in which cells live, disrupting the structural integrity of the cell.
B) Hydrophobic molecules would dissolve in the aqueous environment in which cells live, disrupting the structural integrity of the cell, while hydrophilic molecules could not interact.
C) Hydrophilic molecules would dissolve in the aqueous environment in which cells live, disrupting the structural integrity of the cell, and hydrophobic molecules would be unable to interact with the aqueous environment in which cells live.
D) Hydrophobic molecules would be unable to interact with the aqueous environment in which cells live.
E) Both hydrophilic and hydrophobic molecules would dissolve in the aqueous environment in which cells live, disrupting the structural integrity of the cell.
A) Hydrophilic molecules would dissolve in the aqueous environment in which cells live, disrupting the structural integrity of the cell.
B) Hydrophobic molecules would dissolve in the aqueous environment in which cells live, disrupting the structural integrity of the cell, while hydrophilic molecules could not interact.
C) Hydrophilic molecules would dissolve in the aqueous environment in which cells live, disrupting the structural integrity of the cell, and hydrophobic molecules would be unable to interact with the aqueous environment in which cells live.
D) Hydrophobic molecules would be unable to interact with the aqueous environment in which cells live.
E) Both hydrophilic and hydrophobic molecules would dissolve in the aqueous environment in which cells live, disrupting the structural integrity of the cell.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
Activation energy
A) is the minimum amount of energy needed to get a reaction started and is due to the necessity of collisions between reactants which have enough energy and with the reactants properly oriented and can be lowered by catalysts such as enzymes in biochemical reactions.
B) can be lowered by catalysts such as enzymes in biochemical reactions.
C) is due to the necessity of collisions between reactants which have enough energy and with the reactants properly oriented.
D) is the minimum amount of energy needed to get a reaction started.
E) is the minimum amount of energy needed to get a reaction started and is due to the necessity of collisions between reactants which have enough energy and with the reactants properly oriented.
A) is the minimum amount of energy needed to get a reaction started and is due to the necessity of collisions between reactants which have enough energy and with the reactants properly oriented and can be lowered by catalysts such as enzymes in biochemical reactions.
B) can be lowered by catalysts such as enzymes in biochemical reactions.
C) is due to the necessity of collisions between reactants which have enough energy and with the reactants properly oriented.
D) is the minimum amount of energy needed to get a reaction started.
E) is the minimum amount of energy needed to get a reaction started and is due to the necessity of collisions between reactants which have enough energy and with the reactants properly oriented.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is not one of the four main groups of biomolecules?
A) nucleic acids
B) carbohydrates
C) electrolytes
D) proteins
E) lipids
A) nucleic acids
B) carbohydrates
C) electrolytes
D) proteins
E) lipids

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
Which reaction has X and Y as reactants?
A) XY + W - WY + X
B) XY + AB - XB + AY
C) XY -X + Y
D) X + Y - XY
E) X + YZ - Y + XZ
A) XY + W - WY + X
B) XY + AB - XB + AY
C) XY -X + Y
D) X + Y - XY
E) X + YZ - Y + XZ

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
Which statement is true about carbohydrates?
A) They maybe saturated or unsaturated.
B) They are a component of the cell wall of different types of organisms.
C) They have primary, secondary, tertiary, and quaternary structure.
D) They are usually hydrophobic.
E) They consist of simple sugars which contain carbon, hydrogen, and oxygen in a 2: 1: 2 ratio.
A) They maybe saturated or unsaturated.
B) They are a component of the cell wall of different types of organisms.
C) They have primary, secondary, tertiary, and quaternary structure.
D) They are usually hydrophobic.
E) They consist of simple sugars which contain carbon, hydrogen, and oxygen in a 2: 1: 2 ratio.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
Which reaction is incorrectly matched with its name?
A) Double exchange: AB + CD - AD + CB
B) Hydrolysis: A + B - AB + H2O
C) Synthesis: A + B - AB
D) Single exchange: AB + C - AC + B
E) Decomposition: AB - A + B
A) Double exchange: AB + CD - AD + CB
B) Hydrolysis: A + B - AB + H2O
C) Synthesis: A + B - AB
D) Single exchange: AB + C - AC + B
E) Decomposition: AB - A + B

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
Hydrogen bonds
A) form whenever hydrogen is involved in any covalent or ionic bond.
B) are involved in stabilizing the structure of proteins and nucleic acids and form whenever hydrogen is involved in any covalent or ionic bond.
C) are responsible for the unique properties of water.
D) are responsible for the unique properties of water and are involved in stabilizing the structure of proteins and nucleic acids.
E) are involved in stabilizing the structure of proteins and nucleic acids.
A) form whenever hydrogen is involved in any covalent or ionic bond.
B) are involved in stabilizing the structure of proteins and nucleic acids and form whenever hydrogen is involved in any covalent or ionic bond.
C) are responsible for the unique properties of water.
D) are responsible for the unique properties of water and are involved in stabilizing the structure of proteins and nucleic acids.
E) are involved in stabilizing the structure of proteins and nucleic acids.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
Which type of lipid is incorrectly matched to its description?
A) Mono- , di- , or triglyceride: One, two, or three fatty acids linked to a glycerol molecule
B) Steroid: Made of four fused hydrocarbon rings
C) Phospholipid: An amphipathic lipid found in the plasma membrane of cells
D) Wax: Refers to any lipid which is solid at room temperature
E) Glycolipid: A lipid linked to a carbohydrate
A) Mono- , di- , or triglyceride: One, two, or three fatty acids linked to a glycerol molecule
B) Steroid: Made of four fused hydrocarbon rings
C) Phospholipid: An amphipathic lipid found in the plasma membrane of cells
D) Wax: Refers to any lipid which is solid at room temperature
E) Glycolipid: A lipid linked to a carbohydrate

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
Blood pH is stabilized by carbonic acid (H2CO3) which releases H+ ions to lower pH and bicarbonate (HCO3- ) which absorbs H+ ions to raise pH.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
A molecule of glucose contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. The proper way to write the molecular formula for glucose is 6C12H6O.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
Endergonic reactions make products with a lower final energy than the reactants and use more energy than is released.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
A phosphodiester bond links a fatty acid to a glycerol molecule to form a fat or an oil.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
Describe the relationship between acids, bases, salts, and water, and explain how the pH scale is used to measure acidity and basicity.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
In a polar covalent bond involving hydrogen and oxygen, the hydrogen takes on a partial negative charge while the oxygen takes on a partial positive charge.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
During vigorous exercise, both carbon dioxide and lactic acid enter the blood in increased amounts. Both compounds have the effect of lowering the blood pH. In order to maintain blood pH within the normal range of 7.35- 7.45, we would expect the carbonic acid (H2CO3) portion of the blood buffer system to pick up the extra H+ ions.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
Which level of protein structure can involve hydrogen bonds?
A) primary
B) secondary
C) tertiary
D) primary and secondary
E) secondary and tertiary
A) primary
B) secondary
C) tertiary
D) primary and secondary
E) secondary and tertiary

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
Vitamin deficiencies (not getting enough of a certain vitamin) pose obvious health problems, but an excess of certain vitamins in the system can also be harmful. Explain why you would be far less likely to experience an excess of water- soluble vitamins compared to fat- soluble vitamins in the context of the characteristics of polar and nonpolar substances.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
Compare and contrast ionic bonds, covalent bonds, polar covalent bonds, and hydrogen bonds. Explain how valence electrons are involved in the formation of each.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
Plasma membranes must be in a fluid (liquid) state in order to function properly. Fluidity is temperature- dependent. Bacteria can regulate the specific lipid composition of their plasma membranes. Which of the following statements is true?
A) The proportion of saturated lipids in the plasma membrane will increase with both cooler and warmer growth temperatures.
B) The proportion of unsaturated lipids in the plasma membrane will increase with warmer growth temperatures.
C) The proportion of unsaturated lipids in the plasma membrane will increase with cooler growth temperatures.
D) The proportion of saturated lipids in the plasma membrane will increase with cooler growth temperatures while the proportion of unsaturated lipids will increase with warmer growth temperatures.
E) The proportion of saturated lipids in the plasma membrane will increase with cooler growth temperatures.
A) The proportion of saturated lipids in the plasma membrane will increase with both cooler and warmer growth temperatures.
B) The proportion of unsaturated lipids in the plasma membrane will increase with warmer growth temperatures.
C) The proportion of unsaturated lipids in the plasma membrane will increase with cooler growth temperatures.
D) The proportion of saturated lipids in the plasma membrane will increase with cooler growth temperatures while the proportion of unsaturated lipids will increase with warmer growth temperatures.
E) The proportion of saturated lipids in the plasma membrane will increase with cooler growth temperatures.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
Water (H2O) and carbon dioxide (CO2) are both molecules and compounds.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
Acids increase the H+ concentration in a solution and so lower pH.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
A reversible reaction is one in which the forward and reversible reactions are both possible such as: AB -A + B and A + B -AB.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
List the four types of biomolecules. For each type, name the monomer building block, the type of chemical bond which joins the building block, and give two functions, naming a specific example where appropriate.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
List and describe the four levels of protein structure. Explain how the levels of structure are dependent on each other and describe what types of molecular interactions are involved.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
A cation forms when an atom loses one or more negatively- charged electrons.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
Chaperone proteins
A) escort proteins to the location in a cell where they are needed.
B) ensure that a protein is folded correctly.
C) ensure that amino acids are placed in the correct order when forming a protein.
D) cleave a phosphate from ATP to release energy.
E) ensure that DNA molecules form double- stranded helices.
A) escort proteins to the location in a cell where they are needed.
B) ensure that a protein is folded correctly.
C) ensure that amino acids are placed in the correct order when forming a protein.
D) cleave a phosphate from ATP to release energy.
E) ensure that DNA molecules form double- stranded helices.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
In the human genetic disease sickle cell anemia, a single change in the genetic sequence of the hemoglobin- beta gene results in the amino acid valine being substituted for the amino acid glutamic acid in the beta chain of the hemoglobin protein. Which level(s) of the protein structure will be affected?
A) primary structure
B) secondary structure
C) tertiary structure
D) quaternary structure
E) primary, secondary, tertiary, and quaternary structures
A) primary structure
B) secondary structure
C) tertiary structure
D) quaternary structure
E) primary, secondary, tertiary, and quaternary structures

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
The primary structure of a protein
A) is the linear sequence of amino acids which are held together by peptide bonds and is dictated by genetic sequence.
B) is the linear sequence of amino acids which are held together by peptide bonds and is dictated by genetic sequence and is unique to that protein and lays the foundation for all higher order structure of that protein.
C) is the linear sequence of amino acids which are held together by peptide bonds.
D) is unique to that protein and lays the foundation for all higher order structure of that protein.
E) is dictated by a genetic sequence.
A) is the linear sequence of amino acids which are held together by peptide bonds and is dictated by genetic sequence.
B) is the linear sequence of amino acids which are held together by peptide bonds and is dictated by genetic sequence and is unique to that protein and lays the foundation for all higher order structure of that protein.
C) is the linear sequence of amino acids which are held together by peptide bonds.
D) is unique to that protein and lays the foundation for all higher order structure of that protein.
E) is dictated by a genetic sequence.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck


