Exam 2: Biochemistry Basics
Exam 1: Introduction to Microbiology46 Questions
Exam 2: Biochemistry Basics60 Questions
Exam 3: Introduction to Prokaryotic Cells47 Questions
Exam 4: Introduction to Eukaryotic Cells51 Questions
Exam 5: Genetics55 Questions
Exam 6: Viruses and Prions49 Questions
Exam 7: Fundamentals of Microbial Growth46 Questions
Exam 8: Microbial Metabolism60 Questions
Exam 9: Principles of Infectious Disease and Epidemiology47 Questions
Exam 10: Host-Microbe Interactions and Pathogenesis46 Questions
Exam 11: Innate Immunity60 Questions
Exam 12: Adaptive Immunity60 Questions
Exam 13: Immune System Disorders47 Questions
Exam 14: Vaccines and Biotechnology-Based Diagnostics and Therapeutics47 Questions
Exam 15: Antimicrobial Drugs46 Questions
Exam 16: Respiratory System Infections46 Questions
Exam 17: Skin and Eye Infections47 Questions
Exam 18: Nervous System Infections46 Questions
Exam 19: Digestive System Infections53 Questions
Exam 20: Urinary and Reproductive System Infections46 Questions
Exam 21: Cardiovascular and Lymphatic Infections46 Questions
Select questions type
A compound which stabilizes pH by absorbing or releasing H+ ions is called a(n)
Free
(Multiple Choice)
4.7/5  (39)
(39)
Correct Answer:
B
Which term is incorrectly matched to its description?
Free
(Multiple Choice)
4.8/5  (37)
(37)
Correct Answer:
D
Endergonic reactions make products with a lower final energy than the reactants and use more energy than is released.
Free
(True/False)
4.9/5  (32)
(32)
Correct Answer:
False
Blood pH is stabilized by carbonic acid (H2CO3) which releases H+ ions to lower pH and bicarbonate (HCO3- ) which absorbs H+ ions to raise pH.
(True/False)
4.8/5  (32)
(32)
In a dehydration synthesis reaction, macromolecules are built when is removed to form a covalent bond.
(Multiple Choice)
4.9/5  (29)
(29)
The pictured molecules both contain six carbon atoms, twelve hydrogen atoms, and six oxygen atoms (C6H12O6). However, these atoms are arranged differently in each molecule. What are these molecules called? 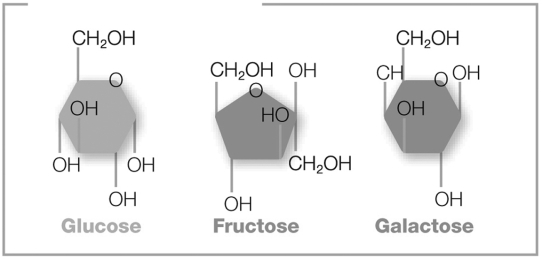
(Multiple Choice)
4.9/5  (38)
(38)
Compare and contrast ionic bonds, covalent bonds, polar covalent bonds, and hydrogen bonds. Explain how valence electrons are involved in the formation of each.
(Essay)
4.8/5  (33)
(33)
List and describe the four levels of protein structure. Explain how the levels of structure are dependent on each other and describe what types of molecular interactions are involved.
(Essay)
4.9/5  (44)
(44)
Which level of protein structure can involve hydrogen bonds?
(Multiple Choice)
4.7/5  (33)
(33)
List the four types of biomolecules. For each type, name the monomer building block, the type of chemical bond which joins the building block, and give two functions, naming a specific example where appropriate.
(Essay)
4.9/5  (39)
(39)
A feature of many of the isotopes that are used in the field of medicine is that the isotopes are radioactive. What does this mean?
(Multiple Choice)
4.8/5  (30)
(30)
The type of bond which links the amino group of one amino acid to the carboxyl group of another amino acid is called a(n)
(Multiple Choice)
4.9/5  (37)
(37)
Compared to a solution with a pH value of 4, a solution with a pH value of 2 has H+ ions.
(Multiple Choice)
4.7/5  (39)
(39)
Showing 1 - 20 of 60
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)