Deck 15: Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/79
Play
Full screen (f)
Deck 15: Thermodynamics
1
What are the SI units of the product of pressure and volume, PV?
A)newton
B)kilogram · meter/second
C)joule
D)meter2
E)newton · second
A)newton
B)kilogram · meter/second
C)joule
D)meter2
E)newton · second
joule
2
Complete the following statement: Walls that separate a system from its surroundings and permit heat to flow through them are called
A)diathermal walls.
B)adiabatic walls.
C)entropic walls.
D)isobaric walls.
E)isochoric walls.
A)diathermal walls.
B)adiabatic walls.
C)entropic walls.
D)isobaric walls.
E)isochoric walls.
diathermal walls.
3
When the gas enclosed beneath the piston shown in the figure receives 2170 J of heat, Q, from its surroundings, it performs 2840 J of work in raising the piston.What is the change in the internal energy of the gas? 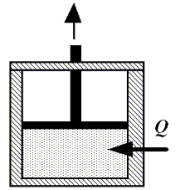
A)+670 J
B)-670 J
C)-5010 J
D)+5010 J
E)zero joules

A)+670 J
B)-670 J
C)-5010 J
D)+5010 J
E)zero joules
-670 J
4
Rick spends four hours researching on the internet and does 1090 J of work.In the process, his internal energy decreases by 2190 J.Determine the value of Q, including the algebraic sign.
A)-1100 J
B)+1100 J
C)-2190 J
D)+3280 J
E)-3280 J
A)-1100 J
B)+1100 J
C)-2190 J
D)+3280 J
E)-3280 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
5
A container is divided into two chambers that are separated by a valve.The left chamber contains one mole of a monatomic ideal gas.The right chamber is evacuated.At some instant, the valve is opened and the gas rushes freely into the right chamber.Which one of the following statements concerning this process is true? 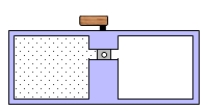
A)Work is done by the gas.
B)The temperature of the gas decreases.
C)The change in the entropy of the gas is zero.
D)The walls of the containing vessel must get colder.
E)The change in the internal energy of the gas is zero.

A)Work is done by the gas.
B)The temperature of the gas decreases.
C)The change in the entropy of the gas is zero.
D)The walls of the containing vessel must get colder.
E)The change in the internal energy of the gas is zero.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
6
Complete the following statement: The first law of thermodynamics states that
A)heat is a form of energy.
B)entropy is a function of state.
C)the entropy of the universe is increasing.
D)the change in the internal energy of a system is given by Q - W.
E)no engine can be more efficient than a Carnot engine operating between the same two temperatures.
A)heat is a form of energy.
B)entropy is a function of state.
C)the entropy of the universe is increasing.
D)the change in the internal energy of a system is given by Q - W.
E)no engine can be more efficient than a Carnot engine operating between the same two temperatures.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
7
If one complete cycle of a reversible process is carried out on a sample of an ideal gas so that its final state is the same as its initial state, which one of the following quantities is the only one which can be non-zero?
A)the change in volume of the sample
B)the net heat absorbed by the sample
C)the change in the entropy of the sample
D)the change in temperature of the sample
E)the change in the internal energy of the sample
A)the change in volume of the sample
B)the net heat absorbed by the sample
C)the change in the entropy of the sample
D)the change in temperature of the sample
E)the change in the internal energy of the sample

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
8
15-1
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.
How much heat is added to the system in the isothermal process of converting all of the water into steam?
A)2.17 × 103 J
B)1.70 × 104 J
C)4.52 × 105 J
D)3.78 × 106 J
E)1.13 × 107 J
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.

How much heat is added to the system in the isothermal process of converting all of the water into steam?
A)2.17 × 103 J
B)1.70 × 104 J
C)4.52 × 105 J
D)3.78 × 106 J
E)1.13 × 107 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
9
15-1
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.
Heat is added to a sample of an ideal monatomic gas.Which one of the following statements is necessarily true?
A)The gas must expand.
B)The gas must do work.
C)The type of change that will occur depends on the conditions of the gas when the heat was added.
D)The gas must change phase.
E)The temperature of the gas must increase.
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.

Heat is added to a sample of an ideal monatomic gas.Which one of the following statements is necessarily true?
A)The gas must expand.
B)The gas must do work.
C)The type of change that will occur depends on the conditions of the gas when the heat was added.
D)The gas must change phase.
E)The temperature of the gas must increase.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
10
15-1
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.
What is the change in the internal energy during this isothermal process?
A)zero joules
B)1.28 × 104 J
C)4.40 × 105 J
D)2.93 × 106 J
E)1.04 × 107 J
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.

What is the change in the internal energy during this isothermal process?
A)zero joules
B)1.28 × 104 J
C)4.40 × 105 J
D)2.93 × 106 J
E)1.04 × 107 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
11
Which one of the following situations is a direct application of the Zeroth Law of Thermodynamics?
A)Block A has twice the temperature of block B before they are brought into contact.Upon contact, heat flows from block A to block B.
B)A sample of gas within a cylinder with a piston is held at constant temperature and pressure while it is allowed to expand.During this process, the gas absorbs heat from its surroundings.
C)The motor of a refrigerator uses electric energy to remove heat from inside the refrigerator and transfer it to the room.
D)A physicist removes energy from a system in her laboratory until it reaches a temperature of 3 × 10-10 K, a temperature very close to (but still greater than)absolute zero.
E)A thermometer is calibrated by placing it in an ice water bath within an adiabatic container until the thermometer is in thermal equilibrium with the ice water.
A)Block A has twice the temperature of block B before they are brought into contact.Upon contact, heat flows from block A to block B.
B)A sample of gas within a cylinder with a piston is held at constant temperature and pressure while it is allowed to expand.During this process, the gas absorbs heat from its surroundings.
C)The motor of a refrigerator uses electric energy to remove heat from inside the refrigerator and transfer it to the room.
D)A physicist removes energy from a system in her laboratory until it reaches a temperature of 3 × 10-10 K, a temperature very close to (but still greater than)absolute zero.
E)A thermometer is calibrated by placing it in an ice water bath within an adiabatic container until the thermometer is in thermal equilibrium with the ice water.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
12
Which one of the following pressure-volume graphs represents an isochoric process? 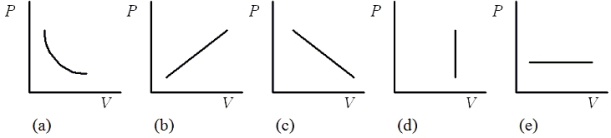
A)(a)
B)(b)
C)(c)
D)(d)
E)(e)

A)(a)
B)(b)
C)(c)
D)(d)
E)(e)

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
13
Enclosed beneath the moveable piston in the drawing is 4.8 moles of a monatomic ideal gas.The gas performs work on the piston as 2300 J of heat are added from the surroundings.During the process, the temperature of the gas decreases by 45 K.How much work does the gas perform? 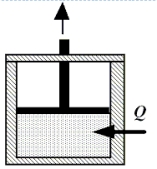
A)5.0 × 103 J
B)3.2 × 103 J
C)1.4 × 103 J
D)6.0 × 102 J
E)4.4 × 103 J

A)5.0 × 103 J
B)3.2 × 103 J
C)1.4 × 103 J
D)6.0 × 102 J
E)4.4 × 103 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
14
An isobaric process is represented on a pressure-volume graph by which one of the following curves?
A)a parabola
B)a hyperbola
C)a vertical line
D)a circle
E)a horizontal line
A)a parabola
B)a hyperbola
C)a vertical line
D)a circle
E)a horizontal line

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
15
A system containing an ideal gas at a constant pressure of 1.42 × 105 Pa gains 2320 J of heat.During the process, the internal energy of the system increases by 2540 J.What is the change in volume of the gas?
A)+1.55 × 10-3 m3
B)-1.55 × 10-3 m3
C)+2.36 × 10-3 m3
D)-2.36 × 10-3 m3
E)zero m3
A)+1.55 × 10-3 m3
B)-1.55 × 10-3 m3
C)+2.36 × 10-3 m3
D)-2.36 × 10-3 m3
E)zero m3

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
16
An ideal gas absorbs 750 J of heat as it performs 625 J of work.What is the resulting change in temperature if there are 1.3 moles of the gas in the system?
A)-8.6 K
B)-4.3 K
C)7.7 K
D)9.6 K
E)23 K
A)-8.6 K
B)-4.3 K
C)7.7 K
D)9.6 K
E)23 K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
17
A thermally isolated sample of an ideal gas at a fixed temperature is confined to one half of a container by an impermeable membrane.The other half of the container is evacuated.The membrane is then pierced and the gas is allowed to expand freely and to double its volume as shown.Which one of the following statements is true concerning this situation? 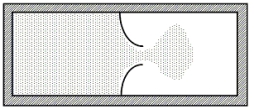
A)The process is reversible.
B)This is an isothermal process.
C)The entropy of the gas decreases.
D)The internal energy of the gas must decrease.
E)The temperature of the gas decreases to one-half of its original value.

A)The process is reversible.
B)This is an isothermal process.
C)The entropy of the gas decreases.
D)The internal energy of the gas must decrease.
E)The temperature of the gas decreases to one-half of its original value.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
18
15-1
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.
How much work is done by this closed system during this isothermal process?
A)8.37 J
B)4.20 × 103 J
C)1.21 × 104 J
D)8.58 × 105 J
E)1.94 × 106 J
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.

How much work is done by this closed system during this isothermal process?
A)8.37 J
B)4.20 × 103 J
C)1.21 × 104 J
D)8.58 × 105 J
E)1.94 × 106 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
19
15-1
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.
Neon is a monatomic gas with a molar heat capacity at constant volume of 12.66 J/(mol · K).Two moles of neon gas enclosed in a constant volume system receive 4250 J of heat.If the gas was initially at 293 K, what is the final temperature of the neon?
A)3400 K
B)400 K
C)460 K
D)520 K
E)600 K
5.00 kg of liquid water is heated to 100.0 °C in a closed system.At this temperature, the density of liquid water is 958 kg/m3.The pressure is maintained at atmospheric pressure of 1.01 × 105 Pa.A moveable piston of negligible weight rests on the surface of the water.The water is then converted to steam by adding an additional amount of heat to the system.When all of the water is converted, the final volume of the steam is 8.50 m3.The latent heat of vaporization of water is 2.26 × 106 J/kg.

Neon is a monatomic gas with a molar heat capacity at constant volume of 12.66 J/(mol · K).Two moles of neon gas enclosed in a constant volume system receive 4250 J of heat.If the gas was initially at 293 K, what is the final temperature of the neon?
A)3400 K
B)400 K
C)460 K
D)520 K
E)600 K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
20
A match ignites within in an oxygen-filled cylinder that has a movable piston.The piston is moved so quickly that no heat escapes.What kind of change is demonstrated in this process?
A)an isobaric change
B)an adiabatic change
C)an isothermal change
D)an isochoric change
E)a change of heat capacity
A)an isobaric change
B)an adiabatic change
C)an isothermal change
D)an isochoric change
E)a change of heat capacity

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
21
A quantity of carbon monoxide gas is slowly compressed adiabatically in an insulated container to one-half of its initial volume.The ratio of the specific heat capacities at constant pressure and constant volume, cP/cV , for carbon dioxide is approximately 1.3.Determine the final pressure of the gas if the initial pressure is 2.0 × 105 Pa.
A)2.0 × 105 Pa
B)2.6 × 105 Pa
C)3.7 × 105 Pa
D)4.9 × 105 Pa
E)5.2 × 105 Pa
A)2.0 × 105 Pa
B)2.6 × 105 Pa
C)3.7 × 105 Pa
D)4.9 × 105 Pa
E)5.2 × 105 Pa

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
22
15-2
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.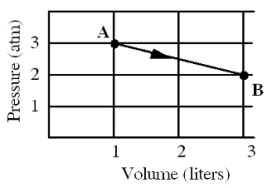
What is the change in the internal energy, in calories, of the gas for this process?
A)zero calories
B)+12 cal
C)-110 cal
D)+110 cal
E)+122 cal
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.

What is the change in the internal energy, in calories, of the gas for this process?
A)zero calories
B)+12 cal
C)-110 cal
D)+110 cal
E)+122 cal

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
23
A fixed amount of ideal gas is compressed adiabatically.Which entry in the table below correctly indicates the sign of the work done, the change in the internal energy, and the heat exchanged with the environment? work done change in internal energy heat exchanged
A)positive negative zero
B)negative zero positive
C)negative negative zero
D)positive positive zero
E)negative positive zero
A)positive negative zero
B)negative zero positive
C)negative negative zero
D)positive positive zero
E)negative positive zero

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
24
15-2
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.
Suppose that the same gas is originally in state A as described above, but its volume is increased isothermally until a new volume of 3.0 liters is reached.Which one of the following statements for this isothermal process is false?
A)The change in the internal energy is zero.
B)The final state of the system will still be B.
C)The work done will be smaller for the isothermal process.
D)The heat added will be smaller for the isothermal process.
E)The heat added for the isothermal process will be equal to the work done.
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.

Suppose that the same gas is originally in state A as described above, but its volume is increased isothermally until a new volume of 3.0 liters is reached.Which one of the following statements for this isothermal process is false?
A)The change in the internal energy is zero.
B)The final state of the system will still be B.
C)The work done will be smaller for the isothermal process.
D)The heat added will be smaller for the isothermal process.
E)The heat added for the isothermal process will be equal to the work done.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
25
During one stage of a reversible process, the temperature of an ideal gas remains constant as its volume is decreased.Which one of the following statements concerning this situation is true?
A)The process is adiabatic.
B)The pressure of the gas decreases in this process.
C)Heat flows out of the gas and into the surroundings.
D)The gas does "positive" work on its surroundings.
E)The average kinetic energy of the gas molecules increases.
A)The process is adiabatic.
B)The pressure of the gas decreases in this process.
C)Heat flows out of the gas and into the surroundings.
D)The gas does "positive" work on its surroundings.
E)The average kinetic energy of the gas molecules increases.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
26
One mole of a monatomic gas at 433 K is reversibly taken to half of its original volume by an isobaric process.How much work is done by the gas?
A)+1800 J
B)-1800 J
C)+3600 J
D)-3600 J
E)-8400 J
A)+1800 J
B)-1800 J
C)+3600 J
D)-3600 J
E)-8400 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
27
An ideal monatomic gas undergoes an adiabatic process; and its internal energy decreases by 50 J.Which pair of choices below is correct for this process? work done heat exchanged
A)50 J by the system zero joules
B)50 J on the system zero joules
C)50 J by the system 100 J supplied
D)zero joules 50 J removed
E)zero joules 50 J added
A)50 J by the system zero joules
B)50 J on the system zero joules
C)50 J by the system 100 J supplied
D)zero joules 50 J removed
E)zero joules 50 J added

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
28
A paddle wheel frictionally adds thermal energy to 4.0 moles of an ideal monatomic gas in a sealed insulated container.The paddle wheel is driven by a cord connected to a falling object as shown in the drawing.How far has the 5.0-kg object fallen when the temperature of the gas increases by 7.50 K? 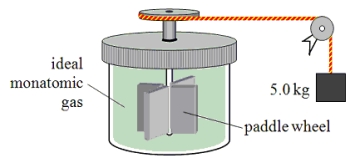
A)7.6 m
B)9.1 m
C)11 m
D)13 m
E)15 m

A)7.6 m
B)9.1 m
C)11 m
D)13 m
E)15 m

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
29
In an isothermal process, 1.59 moles of an ideal gas is compressed to one-fifth of its initial volume at 285 K.What quantity of heat is added to, or removed from, the system during this process?
A)133 J added
B)3020 J added
C)1070 J removed
D)6060 J removed
E)8880 J removed
A)133 J added
B)3020 J added
C)1070 J removed
D)6060 J removed
E)8880 J removed

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
30
A paddle wheel frictionally adds thermal energy to an ideal monatomic gas in a sealed, insulated container.The paddle wheel is driven by a cord connected to a falling object as shown in the drawing.In this experiment, a 5.0-kg object falls through a total distance of 3.0 m and the temperature of the gas is found to increase by 6 C°.Assume that all of the mechanical energy lost by the falling object goes into the gas.How many moles of gas must be present in this container? 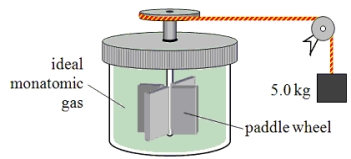
A)2.0
B)3.0
C)4.0
D)5.0
E)6.0

A)2.0
B)3.0
C)4.0
D)5.0
E)6.0

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
31
15-2
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.
How much work, in calories, was done by the gas?
A)zero calories
B)+12 cal
C)-110 cal
D)+110 cal
E)+121 cal
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.

How much work, in calories, was done by the gas?
A)zero calories
B)+12 cal
C)-110 cal
D)+110 cal
E)+121 cal

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
32
15-4
Two moles of an ideal gas have an initial Kelvin temperature Ti and absolute pressure Pi.The gas undergoes a reversible isothermal compression from an initial volume Vi to a final volume 0.5Vi.
Which one of the following expressions represents the final pressure of the gas?
A)0.5Pi
B)Pi/31.7
C)2Pi
D)3.17Pi
E)4Pi
Two moles of an ideal gas have an initial Kelvin temperature Ti and absolute pressure Pi.The gas undergoes a reversible isothermal compression from an initial volume Vi to a final volume 0.5Vi.
Which one of the following expressions represents the final pressure of the gas?
A)0.5Pi
B)Pi/31.7
C)2Pi
D)3.17Pi
E)4Pi

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
33
An ideal gas is taken from state A to state B through process shown on the pressure-volume graph.How much heat is added to the gas in this process? 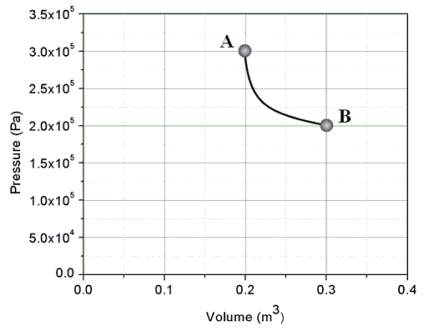
A)zero joules
B)1.0 × 104 J
C)2.4 × 104 J
D)6.0 × 104 J
E)This cannot be determined since n and T are not specified.

A)zero joules
B)1.0 × 104 J
C)2.4 × 104 J
D)6.0 × 104 J
E)This cannot be determined since n and T are not specified.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
34
Two moles of a confined ideal monatomic gas begin at state A on the pressure-volume graph and follow the path shown to state D.If the temperature of the gas at A is 54 K, what is the temperature of the gas at D? 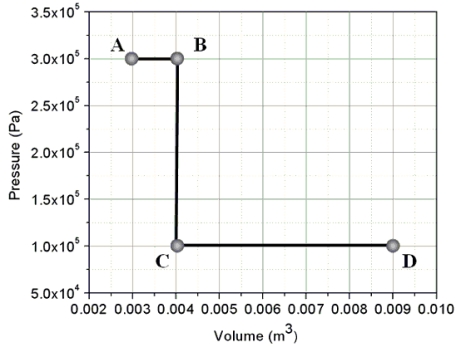
A)32 K
B)46 K
C)54 K
D)60 K
E)78 K

A)32 K
B)46 K
C)54 K
D)60 K
E)78 K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
35
A fixed amount of ideal gas is compressed isothermally.Which entry in the table below correctly depicts the sign of the work done, the change in the internal energy, and the heat exchanged with the environment? work done change in internal energy heat exchanged
A)negative zero negative
B)positive negative zero
C)negative zero positive
D)negative negative zero
E)positive zero positive
A)negative zero negative
B)positive negative zero
C)negative zero positive
D)negative negative zero
E)positive zero positive

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
36
15-2
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.
How much heat, in calories, was exchanged during this process?
A)-110 cal
B)-12 cal
C)zero calories
D)+121 cal
E)+231 cal
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.

How much heat, in calories, was exchanged during this process?
A)-110 cal
B)-12 cal
C)zero calories
D)+121 cal
E)+231 cal

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
37
15-3
An ideal monatomic gas expands isobarically from state A to state B.It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.
How much work is done on the gas in going from B to C?
A)2.5 × 106 J
B)5.5 × 106 J
C)4.5 × 106 J
D)6.5 × 106 J
E)8.0 × 106 J
An ideal monatomic gas expands isobarically from state A to state B.It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.

How much work is done on the gas in going from B to C?
A)2.5 × 106 J
B)5.5 × 106 J
C)4.5 × 106 J
D)6.5 × 106 J
E)8.0 × 106 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
38
Beneath the moveable piston in the drawing,2.25 moles of a monatomic ideal gas is enclosed at 314 K.The initial volume of the gas is 3.0 m3.The gas is compressed isothermally to a final volume of 1.0 m3.How much heat is removed from the gas during this process? 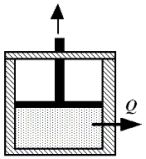
A)-6450 J
B)-4300 J
C)-2900 J
D)-1450 J
E)zero joules

A)-6450 J
B)-4300 J
C)-2900 J
D)-1450 J
E)zero joules

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
39
15-3
An ideal monatomic gas expands isobarically from state A to state B.It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.
How much work is done by the gas in expanding isobarically from A to B?
A)1 × 103 J
B)2 × 103 J
C)3 × 103 J
D)4 × 103 J
E)5 × 103 J
An ideal monatomic gas expands isobarically from state A to state B.It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.

How much work is done by the gas in expanding isobarically from A to B?
A)1 × 103 J
B)2 × 103 J
C)3 × 103 J
D)4 × 103 J
E)5 × 103 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
40
15-3
An ideal monatomic gas expands isobarically from state A to state B.It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.
What is the temperature of the gas when it is in state B?
A)437 K
B)573 K
C)927 K
D)1200 K
E)1473 K
An ideal monatomic gas expands isobarically from state A to state B.It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.

What is the temperature of the gas when it is in state B?
A)437 K
B)573 K
C)927 K
D)1200 K
E)1473 K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
41
Determine the quantity of heat added to 2.8 moles of the ideal gas argon if the temperature increases from 75 °C to 225 °C during an isobaric process.Note: The molar specific heats of argon are CV = 3.0 cal/K·mol and CP = 5.0 cal/K·mol.
A)2600 cal
B)2100 cal
C)1600 cal
D)1100 cal
E)750 cal
A)2600 cal
B)2100 cal
C)1600 cal
D)1100 cal
E)750 cal

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
42
15-5
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
Determine the Carnot efficiency of this engine.
A)0.15
B)0.34
C)0.50
D)0.67
E)0.81
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
Determine the Carnot efficiency of this engine.
A)0.15
B)0.34
C)0.50
D)0.67
E)0.81

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
43
Which one of the following statements is not a consequence of the second law of thermodynamics?
A)The efficiency of any engine is less than 100%.
B)The natural direction of heat flow is from hot to cold.
C)A motor can operate using the atmosphere as a heat reservoir.
D)Only reversible processes have a zero net entropy change for the universe.
E)There is zero net entropy change for the universe in the operation of a real refrigerator.
A)The efficiency of any engine is less than 100%.
B)The natural direction of heat flow is from hot to cold.
C)A motor can operate using the atmosphere as a heat reservoir.
D)Only reversible processes have a zero net entropy change for the universe.
E)There is zero net entropy change for the universe in the operation of a real refrigerator.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
44
A Carnot engine operates between hot and cold reservoirs with temperatures 527 °C and -73.0 °C, respectively.If the engine performs 1000.0 J of work per cycle, how much heat is extracted per cycle from the hot reservoir?
A)878 J
B)1333 J
C)1163 J
D)1527 J
E)2010 J
A)878 J
B)1333 J
C)1163 J
D)1527 J
E)2010 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
45
What is the maximum possible efficiency of an engine operating between the boiling and freezing points of water at sea level?
A)0.27
B)0.49
C)0.61
D)0.85
E)1.0
A)0.27
B)0.49
C)0.61
D)0.85
E)1.0

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
46
15-4
Two moles of an ideal gas have an initial Kelvin temperature Ti and absolute pressure Pi.The gas undergoes a reversible isothermal compression from an initial volume Vi to a final volume 0.5Vi.
What is the change in entropy of the sample?
A)+8.31 (ln 2)J/K
B)-8.31 (ln 2)J/K
C)+16.62 (ln 2)J/K
D)-16.62 (ln 2)J/K
E)zero J/K
Two moles of an ideal gas have an initial Kelvin temperature Ti and absolute pressure Pi.The gas undergoes a reversible isothermal compression from an initial volume Vi to a final volume 0.5Vi.
What is the change in entropy of the sample?
A)+8.31 (ln 2)J/K
B)-8.31 (ln 2)J/K
C)+16.62 (ln 2)J/K
D)-16.62 (ln 2)J/K
E)zero J/K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
47
A Carnot heat engine is to be designed with an efficiency of 81%.If the low temperature reservoir is 25 °C, what is the temperature of the "hot" reservoir?
A)45 °C
B)430 °C
C)850 °C
D)1300 °C
E)1700 °C
A)45 °C
B)430 °C
C)850 °C
D)1300 °C
E)1700 °C

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
48
What change in temperature occurs when 1600 J of heat are removed from 3.0 moles of monatomic gas under constant pressure?
A)-8.1 K
B)-12 K
C)-15 K
D)-26 K
E)-38 K
A)-8.1 K
B)-12 K
C)-15 K
D)-26 K
E)-38 K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
49
A gasoline engine with an efficiency of 0.40 generates 1800 W of power.If a liter of gasoline has an energy content of 3.7 × 107 J, how many liters of gasoline does the engine consume each hour?
A)0.36
B)0.44
C)1.4
D)2.8
E)6.9
A)0.36
B)0.44
C)1.4
D)2.8
E)6.9

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
50
Which one of the following statements is true concerning the ratio of the molar heat capacities Cp/Cv for an ideal gas?
A)The ratio is always 1.
B)The ratio is always less than 1.
C)The ratio is always greater than 1.
D)The ratio is sometimes less than 1.
E)The ratio is sometimes greater than 1.
A)The ratio is always 1.
B)The ratio is always less than 1.
C)The ratio is always greater than 1.
D)The ratio is sometimes less than 1.
E)The ratio is sometimes greater than 1.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
51
A Carnot engine has a heat input of 3700 J and heat exhaust of 1400 J.What is the ratio of the Kelvin temperature of the hot reservoir to that of the cold reservoir, TH/TC?
A)3.6
B)0.38
C)1.6
D)0.62
E)2.6
A)3.6
B)0.38
C)1.6
D)0.62
E)2.6

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
52
Under which one of the following conditions would a Carnot heat engine be 100% efficient?
A)The engine discharges heat at 0 K.
B)The engine uses no heat.
C)The engine does no work.
D)The engine can be operated in reverse.
E)The engine uses an ideal gas.
A)The engine discharges heat at 0 K.
B)The engine uses no heat.
C)The engine does no work.
D)The engine can be operated in reverse.
E)The engine uses an ideal gas.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
53
An engine is used to lift a 2700-kg truck to a height of 3.0 m at constant speed.In the lifting process, the engine received 4.18 × 105 J of heat from the fuel burned in its interior.What is the efficiency of the engine?
A)0.19
B)0.24
C)0.29
D)0.34
E)0.39
A)0.19
B)0.24
C)0.29
D)0.34
E)0.39

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
54
15-5
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
What is the actual efficiency of this engine?
A)0.15
B)0.34
C)0.50
D)0.67
E)0.81
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
What is the actual efficiency of this engine?
A)0.15
B)0.34
C)0.50
D)0.67
E)0.81

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
55
In a reversible heat engine, one mole of an ideal gas is carried through a circular cycle beginning and ending at point A as shown in the figure.Which one of the following statements concerning this system is false? 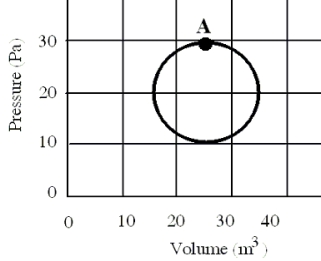
A)The entropy must increase in one cycle.
B)The heat added in one cycle must be 314 J.
C)The work done in completing one cycle is 314 J.
D)The change in internal energy for one cycle is zero joules.
E)The internal energy for this system is dependent on its state.

A)The entropy must increase in one cycle.
B)The heat added in one cycle must be 314 J.
C)The work done in completing one cycle is 314 J.
D)The change in internal energy for one cycle is zero joules.
E)The internal energy for this system is dependent on its state.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
56
Two engines are identical except that engine A is two years old and engine B is new.If engine A does 15% less work than engine B under identical operating conditions, what is the ratio of the efficiencies of the two engines, eA/eB?
A)0.10
B)0.15
C)0.85
D)0.90
E)1.0
A)0.10
B)0.15
C)0.85
D)0.90
E)1.0

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
57
The ratio of the molar specific heat capacity at constant pressure to that at constant volume, , for diatomic hydrogen gas is 7/5.In an adiabatic compression, the gas, originally at atmospheric pressure, is compressed from an original volume of 0.30 m3 to 0.10 m3.What is the final pressure of the gas?
A)2.0 × 105 Pa
B)2.7 × 105 Pa
C)3.1 × 105 Pa
D)3.8 × 105 Pa
E)4.7 × 105 Pa
A)2.0 × 105 Pa
B)2.7 × 105 Pa
C)3.1 × 105 Pa
D)3.8 × 105 Pa
E)4.7 × 105 Pa

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
58
A heat engine operates in a Carnot cycle between reservoirs of temperatures 1000 K and 400 K.It is found to discharge 20 J of heat per cycle to the cold reservoir.What is the work output per cycle?
A)10 J
B)20 J
C)30 J
D)40 J
E)50 J
A)10 J
B)20 J
C)30 J
D)40 J
E)50 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
59
15-4
Two moles of an ideal gas have an initial Kelvin temperature Ti and absolute pressure Pi.The gas undergoes a reversible isothermal compression from an initial volume Vi to a final volume 0.5Vi.
How much heat is exchanged with the environment? If heat is exchanged, is it absorbed or released?
A)PiVi (ln 2), released
B)PiVi (ln 2), absorbed
C)(0.5)PiVi, released
D)(0.5)PiVi, absorbed
E)zero
Two moles of an ideal gas have an initial Kelvin temperature Ti and absolute pressure Pi.The gas undergoes a reversible isothermal compression from an initial volume Vi to a final volume 0.5Vi.
How much heat is exchanged with the environment? If heat is exchanged, is it absorbed or released?
A)PiVi (ln 2), released
B)PiVi (ln 2), absorbed
C)(0.5)PiVi, released
D)(0.5)PiVi, absorbed
E)zero

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
60
Which one of the following statements best describes the operation of a heat engine?
A)A heat engine uses input heat to perform work and rejects excess heat to a lower temperature reservoir.
B)A heat engine performs work and generates an equal amount of heat in a cyclic process.
C)A heat engine transfers heat from a lower temperature reservoir to a higher temperature reservoir through work performed on the system.
D)A heat engine transfers heat from a higher temperature reservoir to a lower temperature reservoir through work performed on the system.
E)A heat engine decreases the entropy of the universe by generating an equal amount of heat and work.
A)A heat engine uses input heat to perform work and rejects excess heat to a lower temperature reservoir.
B)A heat engine performs work and generates an equal amount of heat in a cyclic process.
C)A heat engine transfers heat from a lower temperature reservoir to a higher temperature reservoir through work performed on the system.
D)A heat engine transfers heat from a higher temperature reservoir to a lower temperature reservoir through work performed on the system.
E)A heat engine decreases the entropy of the universe by generating an equal amount of heat and work.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
61
15-5
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
If the coefficient of performance for a refrigerator is 5.0 and 72 J of work are done on the system, how much heat is rejected to the room?
A)390 J
B)430 J
C)360 J
D)310 J
E)530 J
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
If the coefficient of performance for a refrigerator is 5.0 and 72 J of work are done on the system, how much heat is rejected to the room?
A)390 J
B)430 J
C)360 J
D)310 J
E)530 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
62
15-7
An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A.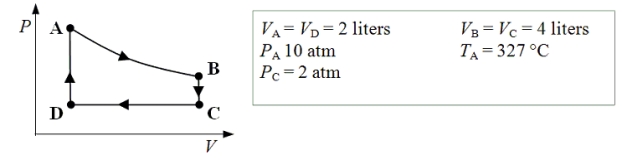
What is the pressure of the gas when it is in state B?
A)5 atm
B)10 atm
C)20 atm
D)25 atm
E)30 atm
An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A.
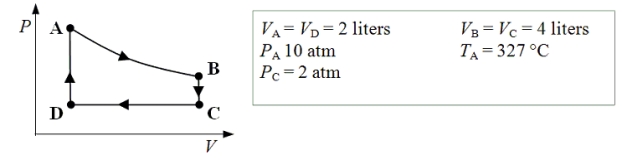
What is the pressure of the gas when it is in state B?
A)5 atm
B)10 atm
C)20 atm
D)25 atm
E)30 atm

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
63
15-5
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
Determine the change in entropy of the cold reservoir.
A)-1.0 × 102 J/K
B)+1.0 × 102 J/K
C)-1.8 × 102 J/K
D)+1.8 × 102 J/K
E)-2.0 × 102 J/K
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
Determine the change in entropy of the cold reservoir.
A)-1.0 × 102 J/K
B)+1.0 × 102 J/K
C)-1.8 × 102 J/K
D)+1.8 × 102 J/K
E)-2.0 × 102 J/K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
64
15-6
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
In which one of these processes will there be no net change in the entropy of the system?
A)the growth of a microorganism
B)the fusion of a crystalline solid
C)the heating of water in an open container
D)the combustion of fuel in a machine engine
E)the evaporation and condensation of benzene in a closed vessel
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
In which one of these processes will there be no net change in the entropy of the system?
A)the growth of a microorganism
B)the fusion of a crystalline solid
C)the heating of water in an open container
D)the combustion of fuel in a machine engine
E)the evaporation and condensation of benzene in a closed vessel

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
65
15-6
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
A block that slides on a rough surface slows down and eventually stops.The reverse process never occurs.That is, a block at rest never begins to move and accelerate on a rough surface without the action of an external agent.The second situation is forbidden because it would violate
A)conservation of total energy.
B)conservation of momentum.
C)the first law of thermodynamics.
D)the second law of thermodynamics.
E)both the first and second laws of thermodynamics.
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
A block that slides on a rough surface slows down and eventually stops.The reverse process never occurs.That is, a block at rest never begins to move and accelerate on a rough surface without the action of an external agent.The second situation is forbidden because it would violate
A)conservation of total energy.
B)conservation of momentum.
C)the first law of thermodynamics.
D)the second law of thermodynamics.
E)both the first and second laws of thermodynamics.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
66
What is the ratio of the internal energy of the gas in state C to that in state A?
A)2/1
B)1/2
C)130/327
D)240/600
E)600/240
A)2/1
B)1/2
C)130/327
D)240/600
E)600/240

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
67
15-5
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
A heat pump extracts 7.0 × 106 J of heat per hour from a well at 280 K and delivers its output heat into a house at 320 K.If the heat pump uses an ideal Carnot cycle in its operation, what minimum work must be supplied to the heat pump per hour?
A)8.6 × 105 J
B)8.0 × 106 J
C)7.7 × 106 J
D)1.0 × 106 J
E)4.7 × 107 J
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
A heat pump extracts 7.0 × 106 J of heat per hour from a well at 280 K and delivers its output heat into a house at 320 K.If the heat pump uses an ideal Carnot cycle in its operation, what minimum work must be supplied to the heat pump per hour?
A)8.6 × 105 J
B)8.0 × 106 J
C)7.7 × 106 J
D)1.0 × 106 J
E)4.7 × 107 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
68
In an isothermal and reversible process, 945 J of heat is removed from a system and transferred to the surroundings.The temperature is 314 K.What is the change in entropy of the system?
A)-3.01 J/K
B)+3.01 J/K
C)-0.332 J/K
D)+0.332 J/K
E)+2.97 × 105 J/K
A)-3.01 J/K
B)+3.01 J/K
C)-0.332 J/K
D)+0.332 J/K
E)+2.97 × 105 J/K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
69
15-6
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
Which one of the following statements is true concerning this process?
A)The water gains entropy in accord with the second law of thermodynamics.
B)The water loses entropy so the process violates the second law of thermodynamics.
C)The water gains entropy, but the air outside the refrigerator loses entropy in accord with the second law of thermodynamics.
D)Both the water and the air outside the refrigerator lose entropy, but the universe gains entropy in accord with the second law of thermodynamics.
E)The water loses entropy, but the air outside the refrigerator gains entropy in accord with the second law of thermodynamics.
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
Which one of the following statements is true concerning this process?
A)The water gains entropy in accord with the second law of thermodynamics.
B)The water loses entropy so the process violates the second law of thermodynamics.
C)The water gains entropy, but the air outside the refrigerator loses entropy in accord with the second law of thermodynamics.
D)Both the water and the air outside the refrigerator lose entropy, but the universe gains entropy in accord with the second law of thermodynamics.
E)The water loses entropy, but the air outside the refrigerator gains entropy in accord with the second law of thermodynamics.

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
70
15-5
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
A heat pump is found to remove 2400 J of heat from the exterior of a house and deliver 3500 J of heat to the interior of the house.What is the coefficient of performance of the heat pump?
A)1.5
B)3.2
C)2.7
D)3.9
E)2.2
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
A heat pump is found to remove 2400 J of heat from the exterior of a house and deliver 3500 J of heat to the interior of the house.What is the coefficient of performance of the heat pump?
A)1.5
B)3.2
C)2.7
D)3.9
E)2.2

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
71
15-6
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
How much heat is extracted from the water in this process?
A)48 000 J
B)170 000 J
C)400 000 J
D)550 000 J
E)348 000 J
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
How much heat is extracted from the water in this process?
A)48 000 J
B)170 000 J
C)400 000 J
D)550 000 J
E)348 000 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
72
A 1.00-kg sample of steam at 100.0 °C condenses to water at 100.0 °C.What is the entropy change of the sample if the heat of vaporization of water is 2.26 × 106 J/kg?
A)-6.06 × 103 J/K
B)+6.06 × 103 J/K
C)-2.26 × 104 J/K
D)+2.26 × 104 J/K
E)zero J/K
A)-6.06 × 103 J/K
B)+6.06 × 103 J/K
C)-2.26 × 104 J/K
D)+2.26 × 104 J/K
E)zero J/K

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
73
15-6
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
Which of the following samples exhibits the highest degree of entropy?
A)a diamond crystal
B)ammonia vapor
C)a block of graphite
D)a block of paraffin
E)liquid oxygen
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
Which of the following samples exhibits the highest degree of entropy?
A)a diamond crystal
B)ammonia vapor
C)a block of graphite
D)a block of paraffin
E)liquid oxygen

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
74
15-7
An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A.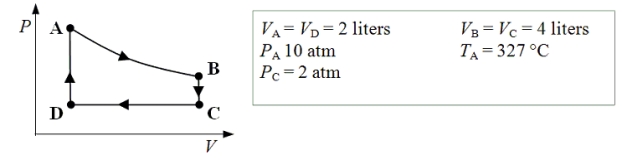
What is the internal energy of the gas at point B?
A)1 × 103 J
B)2 × 103 J
C)3 × 103 J
D)4 × 103 J
E)5 × 103 J
An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A.
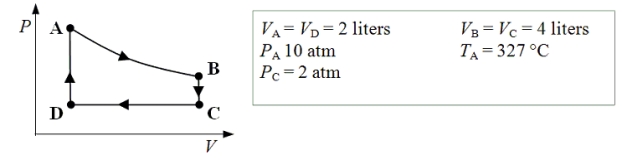
What is the internal energy of the gas at point B?
A)1 × 103 J
B)2 × 103 J
C)3 × 103 J
D)4 × 103 J
E)5 × 103 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
75
What is the net amount of work done after one complete cycle?
A)zero joules
B)20 J
C)40 J
D)1000 J
E)1340 J
A)zero joules
B)20 J
C)40 J
D)1000 J
E)1340 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
76
15-6
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
What is the minimum amount of electrical energy required by the refrigerator to carry out this process if it operates between reservoirs at temperatures of 20.0 °C and -20.0 °C?
A)63 000 J
B)77 000 J
C)87 000 J
D)348 000 J
E)549 000 J
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
What is the minimum amount of electrical energy required by the refrigerator to carry out this process if it operates between reservoirs at temperatures of 20.0 °C and -20.0 °C?
A)63 000 J
B)77 000 J
C)87 000 J
D)348 000 J
E)549 000 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
77
How much work is done on the gas as it is compressed isobarically from state C to state D?
A)zero joules
B)50 J
C)100 J
D)200 J
E)400 J
A)zero joules
B)50 J
C)100 J
D)200 J
E)400 J

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
78
What is the temperature of the gas when it is in state C?
A)-33 °C
B)130 °C
C)327 °C
D)817 °C
E)1500 °C
A)-33 °C
B)130 °C
C)327 °C
D)817 °C
E)1500 °C

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck
79
15-6
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
Which one of the following processes represents a decrease in entropy?
A)the melting of ice
B)the sublimation of carbon dioxide
C)the evaporation of perfume
D)the vaporization of liquid helium
E)the condensation of steam on a kitchen window
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
Which one of the following processes represents a decrease in entropy?
A)the melting of ice
B)the sublimation of carbon dioxide
C)the evaporation of perfume
D)the vaporization of liquid helium
E)the condensation of steam on a kitchen window

Unlock Deck
Unlock for access to all 79 flashcards in this deck.
Unlock Deck
k this deck


