Exam 15: Thermodynamics
Exam 1: Introduction and Mathematical Concepts67 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum65 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena51 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits99 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Reflection of Light: Mirrors42 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity62 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom73 Questions
Exam 31: Nuclear Physics and Radioactivity33 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles43 Questions
Select questions type
A fixed amount of ideal gas is compressed isothermally.Which entry in the table below correctly depicts the sign of the work done, the change in the internal energy, and the heat exchanged with the environment? work done change in internal energy heat exchanged
Free
(Multiple Choice)
4.8/5  (31)
(31)
Correct Answer:
A
Two moles of a confined ideal monatomic gas begin at state A on the pressure-volume graph and follow the path shown to state D.If the temperature of the gas at A is 54 K, what is the temperature of the gas at D? 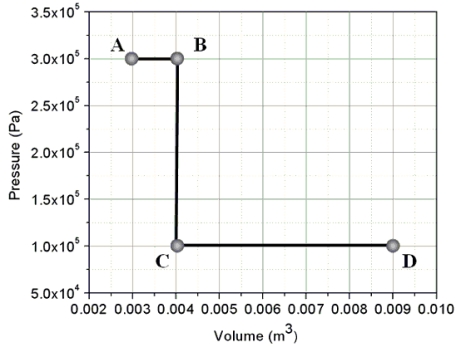
Free
(Multiple Choice)
4.8/5  (35)
(35)
Correct Answer:
C
15-3
An ideal monatomic gas expands isobarically from state A to state B.It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.  -How much work is done on the gas in going from B to C?
-How much work is done on the gas in going from B to C?
Free
(Multiple Choice)
4.8/5  (27)
(27)
Correct Answer:
B
Two engines are identical except that engine A is two years old and engine B is new.If engine A does 15% less work than engine B under identical operating conditions, what is the ratio of the efficiencies of the two engines, eA/eB?
(Multiple Choice)
4.9/5  (35)
(35)
Which one of the following situations is a direct application of the Zeroth Law of Thermodynamics?
(Multiple Choice)
4.8/5  (19)
(19)
15-5
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
-Determine the change in entropy of the cold reservoir.
(Multiple Choice)
4.9/5  (34)
(34)
15-2
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph. 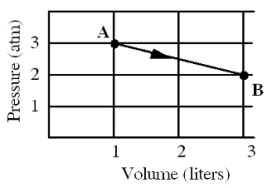 -Suppose that the same gas is originally in state A as described above, but its volume is increased isothermally until a new volume of 3.0 liters is reached.Which one of the following statements for this isothermal process is false?
-Suppose that the same gas is originally in state A as described above, but its volume is increased isothermally until a new volume of 3.0 liters is reached.Which one of the following statements for this isothermal process is false?
(Multiple Choice)
4.8/5  (31)
(31)
15-4
Two moles of an ideal gas have an initial Kelvin temperature Ti and absolute pressure Pi.The gas undergoes a reversible isothermal compression from an initial volume Vi to a final volume 0.5Vi.
-How much heat is exchanged with the environment? If heat is exchanged, is it absorbed or released?
(Multiple Choice)
4.8/5  (35)
(35)
Which one of the following statements best describes the operation of a heat engine?
(Multiple Choice)
4.7/5  (29)
(29)
A heat engine operates in a Carnot cycle between reservoirs of temperatures 1000 K and 400 K.It is found to discharge 20 J of heat per cycle to the cold reservoir.What is the work output per cycle?
(Multiple Choice)
4.8/5  (37)
(37)
What is the ratio of the internal energy of the gas in state C to that in state A?
(Multiple Choice)
4.8/5  (35)
(35)
In an isothermal and reversible process, 945 J of heat is removed from a system and transferred to the surroundings.The temperature is 314 K.What is the change in entropy of the system?
(Multiple Choice)
4.7/5  (31)
(31)
15-3
An ideal monatomic gas expands isobarically from state A to state B.It is then compressed isothermally from state B to state C and finally cooled at constant volume until it returns to its initial state A.  -What is the temperature of the gas when it is in state B?
-What is the temperature of the gas when it is in state B?
(Multiple Choice)
4.8/5  (30)
(30)
A 1.00-kg sample of steam at 100.0 °C condenses to water at 100.0 °C.What is the entropy change of the sample if the heat of vaporization of water is 2.26 × 106 J/kg?
(Multiple Choice)
4.7/5  (38)
(38)
15-7
An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A. 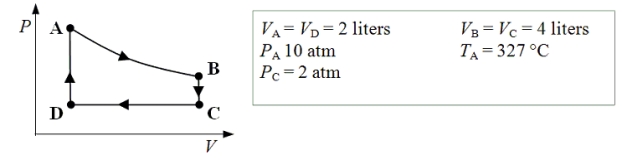 -What is the internal energy of the gas at point B?
-What is the internal energy of the gas at point B?
(Multiple Choice)
4.9/5  (37)
(37)
What are the SI units of the product of pressure and volume, PV?
(Multiple Choice)
4.9/5  (35)
(35)
15-6
A container holding 1.2 kg of water at 20.0 °C is placed in a freezer that is kept at -20.0 °C.The water freezes and comes into thermal equilibrium with the interior of the freezer.
-Which of the following samples exhibits the highest degree of entropy?
(Multiple Choice)
4.8/5  (34)
(34)
Enclosed beneath the moveable piston in the drawing is 4.8 moles of a monatomic ideal gas.The gas performs work on the piston as 2300 J of heat are added from the surroundings.During the process, the temperature of the gas decreases by 45 K.How much work does the gas perform? 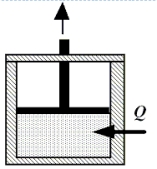
(Multiple Choice)
4.7/5  (42)
(42)
Complete the following statement: The first law of thermodynamics states that
(Multiple Choice)
4.8/5  (33)
(33)
15-5
A heat engine operates between a hot reservoir at 1500 K and a cold reservoir at 500 K.During each cycle, 1.0 × 105 J of heat is removed from the hot reservoir and 5.0 × 104 J of work is performed.
-If the coefficient of performance for a refrigerator is 5.0 and 72 J of work are done on the system, how much heat is rejected to the room?
(Multiple Choice)
4.8/5  (30)
(30)
Showing 1 - 20 of 79
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)