Deck 14: The Ideal Gas Law and Kinetic Theory
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/55
Play
Full screen (f)
Deck 14: The Ideal Gas Law and Kinetic Theory
1
Note the following six properties: 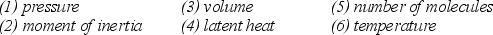 Which four of the listed properties are needed to describe an ideal gas?
Which four of the listed properties are needed to describe an ideal gas?
A)1, 2, 4, 6
B)1, 3, 5, 6
C)1, 3, 4, 6
D)1, 4, 5, 6
E)2, 4, 5, 6
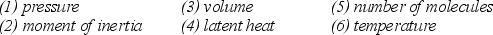 Which four of the listed properties are needed to describe an ideal gas?
Which four of the listed properties are needed to describe an ideal gas?A)1, 2, 4, 6
B)1, 3, 5, 6
C)1, 3, 4, 6
D)1, 4, 5, 6
E)2, 4, 5, 6
1, 3, 5, 6
2
Neon gas at 305 K is confined within a constant volume at a pressure P1.If the gas has a pressure P2 when it is cooled to 125 K, what is the ratio of P2 to P1?
A)0.410
B)0.639
C)0.717
D)1.56
E)2.44
A)0.410
B)0.639
C)0.717
D)1.56
E)2.44
0.410
3
An ideal gas with a fixed number of molecules is maintained at a constant pressure.At 30.0 °C, the volume of the gas is 1.25 m3.What is the volume of the gas when the temperature is increased to 150.0 °C?
A)0.90 m3
B)1.50 m3
C)1.75 m3
D)2.45 m3
E)6.25 m3
A)0.90 m3
B)1.50 m3
C)1.75 m3
D)2.45 m3
E)6.25 m3
1.75 m3
4
Which one of the following statements best explains why gases are not commercially sold by volume?
A)Gas volume is negligible.
B)Gas volume is difficult to measure.
C)Gas volume depends on the type of gas.
D)Gases have comparatively low densities.
E)Gas volume depends on temperature and pressure.
A)Gas volume is negligible.
B)Gas volume is difficult to measure.
C)Gas volume depends on the type of gas.
D)Gases have comparatively low densities.
E)Gas volume depends on temperature and pressure.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
5
In the space between two stars, the temperature of a gas cloud is 12 K; and the density of the gas is 1.2 × 10-8 atom/m3.What is the absolute pressure of the gas?
A)2.0 × 10-30 Pa
B)1.2 × 10-28 Pa
C)2.0 × 10-17 Pa
D)1.2 × 10-6 Pa
E)1.4 × 10-4 Pa
A)2.0 × 10-30 Pa
B)1.2 × 10-28 Pa
C)2.0 × 10-17 Pa
D)1.2 × 10-6 Pa
E)1.4 × 10-4 Pa

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
6
A sample of a monatomic ideal gas is originally at 20 °C.What is the final temperature of the gas if the pressure is doubled and volume is reduced to one-fourth its initial value?
A)900 °C
B)10 °C
C)80 °C
D)(-130 °C)
E)(-260 °C)
A)900 °C
B)10 °C
C)80 °C
D)(-130 °C)
E)(-260 °C)

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
7
A sample of neon gas at 20 °C is confined to a cylinder with a movable piston.The gas is then heated until its pressure is doubled.What is the final temperature of the gas?
A)10 °C
B)40 °C
C)313 °C
D)586 °C
E)This cannot be found since the final and initial volumes are unknown.
A)10 °C
B)40 °C
C)313 °C
D)586 °C
E)This cannot be found since the final and initial volumes are unknown.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
8
An ideal gas is confined within a closed cylinder at a pressure of 2.026 × 105 Pa by a piston.The piston moves until the volume of the gas is reduced to one-ninth of the initial volume.What is the final pressure of the gas when its temperature returns to its initial value?
A)9.117 × 105 Pa
B)6.447 × 105 Pa
C)4.559 × 105 Pa
D)3.102 × 105 Pa
E)1.823 × 106 Pa
A)9.117 × 105 Pa
B)6.447 × 105 Pa
C)4.559 × 105 Pa
D)3.102 × 105 Pa
E)1.823 × 106 Pa

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
9
A silver coin has a mass of 17.75 g.If the atomic mass of silver is 107.868 u, how many silver atoms are in the coin?
A)5.724 × 1022
B)2.158 × 1023
C)4.812 × 1023
D)3.660 × 1024
E)1.390 × 1025
A)5.724 × 1022
B)2.158 × 1023
C)4.812 × 1023
D)3.660 × 1024
E)1.390 × 1025

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
10
Helium gas at 20 °C is confined within a rigid vessel.The gas is then heated until its pressure is doubled.What is the final temperature of the gas?
A)10 °C
B)20 °C
C)40 °C
D)313 °C
E)586 °C
A)10 °C
B)20 °C
C)40 °C
D)313 °C
E)586 °C

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
11
How many molecules are in 0.044 kg of carbon dioxide, CO2? (atomic masses: C = 12 u; O = 16 u)
A)3
B)44
C)2.20 × 1024
D)6.02 × 1023
E)3.85 × 1025
A)3
B)44
C)2.20 × 1024
D)6.02 × 1023
E)3.85 × 1025

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
12
How many moles are in a 0.53-kg sample of sulphur dioxide, SO2? (atomic masses: C = 32 u; O = 16 u)
A)5.2
B)8.3
C)48
D)1.6 × 104
E)5.0 × 1024
A)5.2
B)8.3
C)48
D)1.6 × 104
E)5.0 × 1024

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
13
The density of diamond, a form of carbon, is 3520 kg/m3.If the atomic mass of carbon is 12.011 u, how many carbon atoms are there in a solid diamond sphere with a radius of 0.0125 m?
A)1.15 × 1027 atoms
B)5.76 × 1026 atoms
C)2.88 × 1025 atoms
D)1.44 × 1024 atoms
E)7.21 × 1023 atoms
A)1.15 × 1027 atoms
B)5.76 × 1026 atoms
C)2.88 × 1025 atoms
D)1.44 × 1024 atoms
E)7.21 × 1023 atoms

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
14
Complete the following statement: The atomic mass unit (u) is defined so that 1 u is exactly equal to the mass of
A)a single hydrogen atom.
B)1/4 of a helium molecule.
C)1/16 of an oxygen-16 atom.
D)1/32 of an oxygen molecule.
E)1/12 of a carbon-12 atom.
A)a single hydrogen atom.
B)1/4 of a helium molecule.
C)1/16 of an oxygen-16 atom.
D)1/32 of an oxygen molecule.
E)1/12 of a carbon-12 atom.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
15
An ideal gas at 0 °C is contained within a rigid vessel.The temperature of the gas is increased by 1 C°.What is Pf/Pi, the ratio of the final to initial pressure?
A)273/274
B)274/273
C)1/2
D)1/10
E)1/273
A)273/274
B)274/273
C)1/2
D)1/10
E)1/273

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following statements concerning the mole is false?
A)The mole is related to Avogadro's number.
B)The mole is defined in terms of the carbon-12 isotope.
C)The mole is the SI base unit for expressing the "amount" of a substance.
D)One mole of a substance has the same mass as one mole of any other substance.
E)One mole of a substance contains the same number of particles as one mole of any other substance.
A)The mole is related to Avogadro's number.
B)The mole is defined in terms of the carbon-12 isotope.
C)The mole is the SI base unit for expressing the "amount" of a substance.
D)One mole of a substance has the same mass as one mole of any other substance.
E)One mole of a substance contains the same number of particles as one mole of any other substance.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
17
What is the mass of 3.0 moles of hydrogen molecules, H2? (atomic mass of H = 1 u)
A)0.0060 g
B)0.0030 kg
C)0.0090 kg
D)0.015 kg
E)0.030 kg
A)0.0060 g
B)0.0030 kg
C)0.0090 kg
D)0.015 kg
E)0.030 kg

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
18
Two pure samples of atoms, labeled A and B, contain titanium (Ti) atoms and carbon (C) atoms, respectively.Each sample contains the same number of atoms.What is the ratio of the mass of sample B to that of sample A, mB/mA? Note the following atomic masses: C = 12 u; Ti = 48 u.
A)1.0
B)0.25
C)2.0
D)0.50
E)4.0
A)1.0
B)0.25
C)2.0
D)0.50
E)4.0

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
19
Heat is supplied to a sample of a monatomic ideal gas at 40 °C.It is observed that the gas expands until its volume and pressure are doubled.What is the final temperature of the gas?
A)10 °C
B)20 °C
C)40 °C
D)980 °C
E)1600 °C
A)10 °C
B)20 °C
C)40 °C
D)980 °C
E)1600 °C

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
20
What is the approximate mass of an oxygen molecule, O2, if the atomic mass of oxygen is 15.9994 u?
A)2.657 × 10-26 kg
B)5.313 × 10-26 kg
C)3.200 × 10-25 kg
D)6.400 × 10-25 kg
E)1.927 × 10-23 kg
A)2.657 × 10-26 kg
B)5.313 × 10-26 kg
C)3.200 × 10-25 kg
D)6.400 × 10-25 kg
E)1.927 × 10-23 kg

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
21
Complete the following statement: The pressure exerted by a monatomic, ideal gas on the walls of its containing vessel is a measure of
A)the molecular kinetic energy per unit volume.
B)the average random kinetic energy per molecule.
C)the temperature of the gas, regardless of the volume of the vessel.
D)the total internal energy of the gas, regardless of the volume of the vessel.
E)the momentum per unit volume.
A)the molecular kinetic energy per unit volume.
B)the average random kinetic energy per molecule.
C)the temperature of the gas, regardless of the volume of the vessel.
D)the total internal energy of the gas, regardless of the volume of the vessel.
E)the momentum per unit volume.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
22
Which one of the following factors is directly responsible for the pressure exerted by a confined gas?
A)the atomic mass of the gas
B)the density of the sample of molecules
C)the temperature of the sample of molecules
D)the collision of gas molecules with the sides of the containing vessel
E)the average translational kinetic energy of the molecules
A)the atomic mass of the gas
B)the density of the sample of molecules
C)the temperature of the sample of molecules
D)the collision of gas molecules with the sides of the containing vessel
E)the average translational kinetic energy of the molecules

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
23
A 7.50 × 10-3-m3 bottle contains 0.0200 kg of oxygen gas, O2, at 77.0 °C.What is the pressure exerted on the inner walls of the flask by the oxygen gas? Note: the atomic mass of O is 15.9994 u.
A)8.42 × 104 Pa
B)1.45 × 105 Pa
C)2.43 × 105 Pa
D)4.86 × 105 Pa
E)1.46 × 106 Pa
A)8.42 × 104 Pa
B)1.45 × 105 Pa
C)2.43 × 105 Pa
D)4.86 × 105 Pa
E)1.46 × 106 Pa

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
24
Consider two ideal gases, A and B, at the same temperature.The rms speed of the molecules of gas A is twice that of gas B.How does the molecular mass of A compare to that of B?
A)The molecular mass of A is twice that of B.
B)The molecular mass of A is one half that of B.
C)The molecular mass of A is 1.4 times that of B.
D)The molecular mass of A is one fourth that of B.
E)The molecular mass of A is four times that of B.
A)The molecular mass of A is twice that of B.
B)The molecular mass of A is one half that of B.
C)The molecular mass of A is 1.4 times that of B.
D)The molecular mass of A is one fourth that of B.
E)The molecular mass of A is four times that of B.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
25
An ideal gas is contained in a vessel with a moveable piston.Initially, the gas has a volume of 0.024 m3, an absolute pressure of 1.8 atm, and a temperature of 35.0 °C.The pressure is 0.90 atm when the volume of the container is decreased to 0.012 m3.What is the final temperature of the gas?
A)77 K
B)85 K
C)170 K
D)154 K
E)282 K
A)77 K
B)85 K
C)170 K
D)154 K
E)282 K

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
26
A mixture of two ideal gases A and B is in thermal equilibrium at 600 K.A molecule of A has one-fourth the mass of a molecule of B and the rms speed of molecules of A is 400 m/s.Determine the rms speed of molecules of B.
A)100 m/s
B)200 m/s
C)400 m/s
D)800 m/s
E)1600 m/s
A)100 m/s
B)200 m/s
C)400 m/s
D)800 m/s
E)1600 m/s

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
27
The volume of a carbon dioxide bubble rising in a glass of beer is observed to nearly double as the bubble rises from the bottom to the top of the glass.Why, according to our textbook, does the volume nearly double?
A)The temperature at the bottom is cooler than it is at the top.
B)The amount of carbon dioxide in the bubble increases.
C)The fluid pressure of the beer is greater at the bottom of the glass than at the top.
D)The pressure inside the bubble decreases as it rises.
E)The shape of the glass determines the net force exerted on the bubble.
A)The temperature at the bottom is cooler than it is at the top.
B)The amount of carbon dioxide in the bubble increases.
C)The fluid pressure of the beer is greater at the bottom of the glass than at the top.
D)The pressure inside the bubble decreases as it rises.
E)The shape of the glass determines the net force exerted on the bubble.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
28
At what temperature would one mole of molecular nitrogen (N2) have 7.0 × 103 J of translational kinetic energy? Note: the atomic mass of N is 14 u.
A)130 °C
B)290 °C
C)480 °C
D)560 °C
E)720 °C
A)130 °C
B)290 °C
C)480 °C
D)560 °C
E)720 °C

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
29
A sealed container has a volume of 0.020 m3 and contains 15.0 g of molecular nitrogen (N2), which has a molecular mass of 28.0 u.The gas is at 525 K.What is the absolute pressure of the nitrogen gas?
A)3.9 × 10-19 Pa
B)4.3 × 10-5 Pa
C)1.2 × 105 Pa
D)1.9 × 105 Pa
E)4.3 × 106 Pa
A)3.9 × 10-19 Pa
B)4.3 × 10-5 Pa
C)1.2 × 105 Pa
D)1.9 × 105 Pa
E)4.3 × 106 Pa

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
30
A canister containing 115 kg of an ideal gas has a volume of 6.5 m3.If the gas exerts a pressure of 4.0 × 105 Pa, what is the rms speed of the molecules?
A)260 m/s
B)180 m/s
C)310 m/s
D)390 m/s
E)420 m/s
A)260 m/s
B)180 m/s
C)310 m/s
D)390 m/s
E)420 m/s

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
31
Under which of the following conditions would you expect real gases to approach ideal behavior?
A)low temperature and low pressure
B)high temperature and low pressure
C)low temperature and high pressure
D)high temperature and high pressure
E)high temperature and high density
A)low temperature and low pressure
B)high temperature and low pressure
C)low temperature and high pressure
D)high temperature and high pressure
E)high temperature and high density

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
32
Complete the following statement: The internal energy of an ideal monatomic gas is
A)proportional to the pressure and inversely proportional to the volume of the gas.
B)independent of the number of moles of the gas.
C)proportional to the Kelvin temperature of the gas.
D)dependent on both the pressure and the temperature of the gas.
E)a constant that is independent of pressure, volume or temperature.
A)proportional to the pressure and inversely proportional to the volume of the gas.
B)independent of the number of moles of the gas.
C)proportional to the Kelvin temperature of the gas.
D)dependent on both the pressure and the temperature of the gas.
E)a constant that is independent of pressure, volume or temperature.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
33
A physics student looks into a microscope and observes that small particles suspended in water are moving about in an irregular motion.Which of the following statements is the best explanation for this observation?
A)Water molecules strike the particles giving them the same average kinetic energy as the water.
B)The particles are carried by convection currents in the water.
C)The small particles may be considered a fluid; and thus, move about randomly.
D)The actual motion is regular, but the speeds of particles are too large to observe the regular motion.
E)The particles are moving to be uniformly distributed throughout the volume of the water.
A)Water molecules strike the particles giving them the same average kinetic energy as the water.
B)The particles are carried by convection currents in the water.
C)The small particles may be considered a fluid; and thus, move about randomly.
D)The actual motion is regular, but the speeds of particles are too large to observe the regular motion.
E)The particles are moving to be uniformly distributed throughout the volume of the water.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
34
Complete the following statement: The absolute temperature of an ideal gas is directly proportional to
A)the number of molecules in the sample.
B)the average momentum of a molecule of the gas.
C)the average translational kinetic energy of the gas.
D)the amount of heat required to raise the temperature of the gas by 1 C°.
E)the relative increase in volume of the gas for a temperature increase of 1 C°.
A)the number of molecules in the sample.
B)the average momentum of a molecule of the gas.
C)the average translational kinetic energy of the gas.
D)the amount of heat required to raise the temperature of the gas by 1 C°.
E)the relative increase in volume of the gas for a temperature increase of 1 C°.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
35
Which one of the following properties of a gas is not consistent with kinetic theory?
A)The average speed of the gas molecules is smaller at high temperatures.
B)Gas molecules are widely separated.
C)Gases fill whatever space is available to them.
D)Gas molecules move rapidly in a random fashion.
E)Gas molecules make elastic collisions with the walls of the containing vessel.
A)The average speed of the gas molecules is smaller at high temperatures.
B)Gas molecules are widely separated.
C)Gases fill whatever space is available to them.
D)Gas molecules move rapidly in a random fashion.
E)Gas molecules make elastic collisions with the walls of the containing vessel.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
36
Two moles of a monatomic gas with an rms speed of 294 m/s are contained in a tank that has a volume of 0.12 m3.If each gas particle has a mass of 8.220 × 10-26 kg, what is the absolute pressure of the gas?
A)2.1 × 105 Pa
B)1.1 × 104 Pa
C)4.8 × 104 Pa
D)2.4 × 104 Pa
E)3.0 × 104 Pa
A)2.1 × 105 Pa
B)1.1 × 104 Pa
C)4.8 × 104 Pa
D)2.4 × 104 Pa
E)3.0 × 104 Pa

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
37
The temperature of a monatomic ideal gas with a mass per mole of 0.00750 kg/mol is 298 K.The absolute pressure of the gas is 1.65 × 105 Pa when its volume is 1.21 × 10-3 m3.What is the mass of the gas?
A)9.11 × 10-5 kg
B)2.18 × 10-4 kg
C)4.22 × 10-4 kg
D)6.04 × 10-4 kg
E)2.27 × 10-3 kg
A)9.11 × 10-5 kg
B)2.18 × 10-4 kg
C)4.22 × 10-4 kg
D)6.04 × 10-4 kg
E)2.27 × 10-3 kg

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
38
Complete the following statement: A bicycle tire explodes after lying in the hot afternoon sun.This is an illustration of
A)Charles' law.
B)Boyle's law.
C)Fick's law.
D)the ideal gas law.
E)the Maxwell speed distribution.
A)Charles' law.
B)Boyle's law.
C)Fick's law.
D)the ideal gas law.
E)the Maxwell speed distribution.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
39
Which one of the following statements concerning a collection of gas molecules at a certain temperature is true?
A)All molecules move with the same velocity.
B)Most of the molecules have the same kinetic energy.
C)The lower the temperature, the greater are the molecular speeds.
D)All molecules possess the same momentum.
E)The molecules have a range of kinetic energies.
A)All molecules move with the same velocity.
B)Most of the molecules have the same kinetic energy.
C)The lower the temperature, the greater are the molecular speeds.
D)All molecules possess the same momentum.
E)The molecules have a range of kinetic energies.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
40
A five-liter tank contains 2.00 moles of oxygen gas, O2, at 40 °C.What pressure is exerted on the sides of the tank by the oxygen molecules?
A)83.3 Pa
B)4.01 × 103 Pa
C)1.33 × 105 Pa
D)4.01 × 105 Pa
E)1.04 × 106 Pa
A)83.3 Pa
B)4.01 × 103 Pa
C)1.33 × 105 Pa
D)4.01 × 105 Pa
E)1.04 × 106 Pa

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
41
An automobile tire is inflated to a gauge pressure of 32 lb/in2 at a temperature of -10.0 °C.Under strenuous driving, the tire heats up to 40.0 °C.What is the new gauge pressure if the volume of the tire remains essentially the same? (atmospheric pressure = 14.7 lb/in2)
A)17.5 lb/in2
B)20.6 lb/in2
C)38.0 lb/in2
D)40.9 lb/in2
E)55.6 lb/in2
A)17.5 lb/in2
B)20.6 lb/in2
C)38.0 lb/in2
D)40.9 lb/in2
E)55.6 lb/in2

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
42
A bubble with a volume of 1.0 cm3 forms at the bottom of a lake that is 20.0 m deep.The temperature at the bottom of the lake is 10.0 °C.The bubble rises to the surface where the temperature is 25.0 °C.Assume that the bubble is small enough that its temperature always matches that of its surroundings.What is the volume of the bubble just before it breaks the surface of the water?
A)2.1 cm3
B)2.8 cm3
C)3.1 cm3
D)6.0 cm3
E)7.7 cm3
A)2.1 cm3
B)2.8 cm3
C)3.1 cm3
D)6.0 cm3
E)7.7 cm3

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
43
On a cold day (-3 °C), the gauge pressure on a tire is 2.0 atm.If the tire is heated to 27 °C, without changing its volume, what will the gauge pressure be?
A)2.1 atm
B)2.3 atm
C)2.9 atm
D)3.3 atm
E)27 atm
A)2.1 atm
B)2.3 atm
C)2.9 atm
D)3.3 atm
E)27 atm

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
44
Calculate the rms speed of the carbon dioxide molecules in the air if the temperature is 15.0 °C.Note: The mass of the carbon dioxide molecule is 44.01 u.
A)316 m/s
B)469 m/s
C)378 m/s
D)404 m/s
E)511 m/s
A)316 m/s
B)469 m/s
C)378 m/s
D)404 m/s
E)511 m/s

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
45
How many air molecules are in a room at temperature 23.8 °C and standard pressure if the dimensions of the room are 3.44 m × 3.26 m × 2.28 m?
A)2.08 × 1024
B)4.20 × 1024
C)3.78 × 1025
D)6.32 × 1026
E)8.05 × 1026
A)2.08 × 1024
B)4.20 × 1024
C)3.78 × 1025
D)6.32 × 1026
E)8.05 × 1026

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
46
A flask contains 1.00 mole of oxygen gas, O2, at 0.00 °C and 1.013 × 105 Pa.What is the rms speed of the molecules? Note: the atomic mass of O is 16 u.
A)230 m/s
B)460 m/s
C)651 m/s
D)920 m/s
E)1302 m/s
A)230 m/s
B)460 m/s
C)651 m/s
D)920 m/s
E)1302 m/s

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
47
A gas molecule with a molecular mass of 32.0 u has a speed of 325 m/s.What is the temperature of the gas molecule?
A)72.0 K
B)136 K
C)305 K
D)459 K
E)A temperature cannot be assigned to a single molecule.
A)72.0 K
B)136 K
C)305 K
D)459 K
E)A temperature cannot be assigned to a single molecule.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
48
A lower tank contains water while an upper tank contains air with a negligible concentration of water vapor.A tube with a circular cross section and radius 0.050 m is inserted between the two tanks.The length of the tube is 0.25 m.The diffusion coefficient of water vapor through air is 2.4 × 10-5 m2/s.The concentration of water vapor just above the surface of the water is 1.7 × 10-2 kg/m3.If the water vapor is removed from the upper tank so that the concentration there remains nearly zero, what mass of water vapor diffuses through the tube each hour?
A)4.6 × 10-5 kg
B)2.0 × 10-6 kg
C)7.1 × 10-7 kg
D)1.3 × 10-8 kg
E)8.2 × 10-8 kg
A)4.6 × 10-5 kg
B)2.0 × 10-6 kg
C)7.1 × 10-7 kg
D)1.3 × 10-8 kg
E)8.2 × 10-8 kg

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
49
What is the internal energy of 1.75 kg of helium (atomic mass = 4.00260 u) with a temperature of 100 °C?
A)4.65 × 103 J
B)5.44 × 105 J
C)2.03 × 106 J
D)8.16 × 106 J
E)1.22 × 107 J
A)4.65 × 103 J
B)5.44 × 105 J
C)2.03 × 106 J
D)8.16 × 106 J
E)1.22 × 107 J

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
50
Which one of the following statements concerning diffusion is true?
A)Diffusion occurs only in gases.
B)Diffusion occurs in solids and fluids.
C)Diffusion is described by Boyle's law.
D)The SI unit for the diffusion constant D is m/s2.
E)Diffusion occurs when molecules move from regions of lower concentration to regions of higher concentration.
A)Diffusion occurs only in gases.
B)Diffusion occurs in solids and fluids.
C)Diffusion is described by Boyle's law.
D)The SI unit for the diffusion constant D is m/s2.
E)Diffusion occurs when molecules move from regions of lower concentration to regions of higher concentration.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
51
A concentration difference of a certain solute of 1.0 × 10-2 kg/m3 is maintained between the ends of a tube with a length of 3.5 m and a cross-sectional area of 0.25 m2.When 0.0040 g of the solute is introduced to the tube, it takes 350 minutes for this solute to diffuse through the solvent to the opposite end of the tube.What is the diffusion constant for the solute?
A)2.7 × 10-7 m2/s
B)4.5 × 10-9 m2/s
C)7.5 × 10-10 m2/s
D)6.3 × 10-8 m2/s
E)1.1 × 10-11 m2/s
A)2.7 × 10-7 m2/s
B)4.5 × 10-9 m2/s
C)7.5 × 10-10 m2/s
D)6.3 × 10-8 m2/s
E)1.1 × 10-11 m2/s

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
52
An air bubble 20 m below the surface of a lake has a volume of 0.02 m3.The bubble then rises to the surface.Assuming the temperature of the lake is uniform, what is the volume of the bubble just before it breaks through the surface?
A)0.02 m3
B)0.04 m3
C)0.06 m3
D)0.08 m3
E)0.10 m3
A)0.02 m3
B)0.04 m3
C)0.06 m3
D)0.08 m3
E)0.10 m3

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
53
What is the density of methane, CH4, (molecular mass = 16 u) at STP?
A)0.357 kg/m3
B)0.386 kg/m3
C)0.431 kg/m3
D)0.712 kg/m3
E)0.951 kg/m3
A)0.357 kg/m3
B)0.386 kg/m3
C)0.431 kg/m3
D)0.712 kg/m3
E)0.951 kg/m3

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
54
A tank contains 135 moles of the monatomic gas argon at a temperature of 15.3 °C.How much energy must be added to the gas to increase its temperature to 45.0 °C?
A)2.50 × 103 J
B)3.33 × 104 J
C)5.00 × 104 J
D)5.70 × 105 J
E)7.50 × 106 J
A)2.50 × 103 J
B)3.33 × 104 J
C)5.00 × 104 J
D)5.70 × 105 J
E)7.50 × 106 J

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
55
On a cold day (-3 °C), the gauge pressure on a tire reads 2.0 atm.If the tire is heated to 27 °C, what will be the absolute pressure of the air inside the tire?
A)2.0 atm
B)2.2 atm
C)2.4 atm
D)2.9 atm
E)3.3 atm
A)2.0 atm
B)2.2 atm
C)2.4 atm
D)2.9 atm
E)3.3 atm

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck


